NCERT Solutions for Class-12 Chemistry Chapter-10 Haloalkanes and Haloarenes
NCERT IN-TEXT QUESTIONS
Question 1. Write the structures of the following compounds : (C.B.S.E. Delhi 2010)
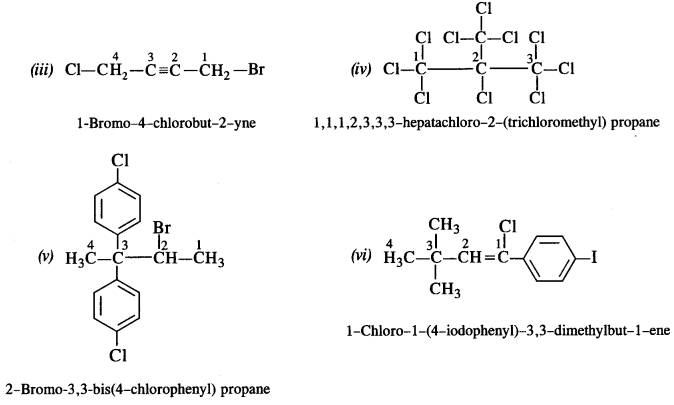
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
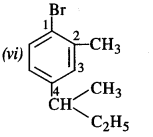
(v) 1-Bromo-4-sec butyl-2-methylbenzene. (C.B.S.E. Sample paper 2011)
Answer:
Question 2. Why is sulphuric acid not used during the reaction of alcohols with KI?
Answer: H2SO4 is an oxidising agent. It oxidises HI produced during the reaction to I2 and thus prevents the reaction between an alcohol and HI to form alkyl iodide. To prevent this, a non¬oxidising acid like H3PO3 is used.
Question 3. Write the structures of different dihalogen derivatives of propane.
Answer: Propane (CH3CH2CH3) has two primary and one secondary hydrogen atoms present. Four isomeric dihalogen derivatives are possible. Let the halogen X be Br.
Question 4. Among the isomeric alkanes of molecular formula C5H12, identify the one which on photochemical chlorination yields
(i) A single monochloride
(ii) Three isomeric monochlorides
(iii) Four isomeric monochlorides.
Answer: The molecular formula C5H12 represents three structural isomers which are chain isomers.
(i) The isomer is symmetrical with four primary (1°) carbon atoms and one quaternary (4°) carbon atom. Since all the hydrogen atoms are equivalent, it will yield only one monochloride upon photochlorination i.e., chlorination carried in the presence of ultra-violet light.
(ii) In the straight chain isomer pentane, there are three groups of equivalent hydrogen atoms. As a result, three isomeric monochlorides are possible
(iii) The branched chain isomer has four types of equivalent hydrogen atoms present. It will give four isomeric monochlorides upon chlorination.
Question 5. Draw the structures of the major monohaloproducts in each of the following reactions:
Answer:
The reaction is carried in the presence of dry acetone upon heating. It is called Finkelstein reaction. In this reaction, I– ion being a stronger nucleophile displaces Br– ion. NaBr formed is insoluble in dry acetone whereas Nal dissolves. This shifts the equilibrium in the forward direction
Under the reaction conditions allylic halogenation will take place. Addition of bromine can be possible in case the reaction is carried at room temperature.
Question 6. Arrange each set of compounds in order of increasing boiling points.
(i)Bromomethane, Bromoform, Chloromethane, Dibromomethane.
(ii)1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Answer:
(i) Chloromethane < Bromomethane < Dibromomethane < Bromoform
The reason is:
(a)for same alkyl group, B.Pt increases with size of halogen atom.
(b)B.Pt increases as number of halogen atoms increase.
(ii)Isopropyl chloride < 1 – Chloropropane < 1 – Chlorobutane
Reason :
(a)For same halogen, B.Pt. increases as size of alkyl group increases.
(b)B.Pt. decreases as branching increases.
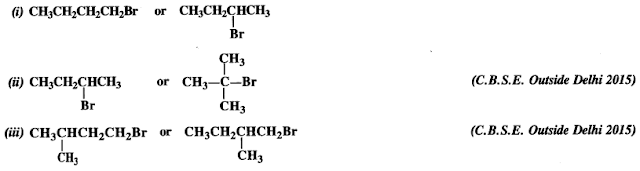
Question 7. Which alkyl halide from the following pairs would you expect to react more rapidly by SN² mechanism ? Explain your answer.
Answer: If the leaving group is the same in different isomers of a particular molecular formula, the reactivity of the isomers towards SN² mechanism decreases with the increase in steric hindrance. In the light of above, the reactivity order in different cases is :
(i) CH3CH2CH2CH2Br is a primary alkyl halide (1°). It is more reactive than the other isomer which is a secondary (2°) alkyl halide because less steric hindrance is caused by primary alkyl group as compared to secondary alkyl group.
(ii)
is a secondary alkyl halide (2°). It is more reactive than the other isomer which is a tertiary alkyl halide (3°). The explanation is the same.
(iii) Here both the isomers are primary alkyl halides (1°). However, the isomer with CH3 group at C2 atom exerts more steric hindrance to the attacking nucleophile at C1 atom as compared to the other isomer in which a CH3 group is attached to C3 atom. It is, therefore, less reactive.
Question 8. In the following pairs of halogen compounds, which compound undergoes reaction faster ? (C.B.S.E. Delhi 2008, Outside Delhi 2010, 2013)
Answer: The reactivity of a particular halogen compound towards SN¹ reaction depends upon the stability of the carbocation formed as a result of ionisation. This is a slow step and is called rate determining step. The order of relative stabilities of different carbocations is in the order : tertiary > secondary > primary. In the light of this, the order of reactivity in the two cases is explained.
1. The isomer (a) is a tertiary alkyl chloride while the other isomer (b) is a secondary alkyl chloride. The isomer (a) is more reactive towards S i reaction since the tertiary carbocation formed in this case is more stable than the secondary carbocation which is likely to be formed in the other case.
2. The isomer (a) is a secondary alkyl chloride while the other isomer (b) is primary in nature. The secondary alkyl chloride (a) is expected to react faster since the secondary carbocation formed is more stable than the primary carbocation which is likely to be formed in the other case.
Question 9. Identify A, B, C, D, E, R and R’ in the following :

Answer

NCERT EXERCISE
Question 1. Name the following compounds according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary) vinyl or aryl halides.
(i) (CH3)2CHCH(C1)CH3 (C.B.S.E. Delhi 2013)
(ii) CH3CH2CH(CH3)CH(C2H5)Cl
(iii) CH3CH2C(CH3)2CH2I
(iv) CH3C(C1)(C2H5)CH2CH3
(v) CH3.C(C2H5)2CH2Br
(vi) CH3CH=C(C1)CH2CH(CH3)2
(vii) CH2=CH-CH2-Br
(viii) CH3CH=CHC(Br)(CH3)2
(ix) m-C1CH2C6H4CH2C(CH3)3
(x) o-BrC6H4CH(CH3)CH2CH3
(xi) (CH3)3CCH2CH(Br)C5H5
(xii) p-ClC6H4CH2CH(CH3)2
Answer: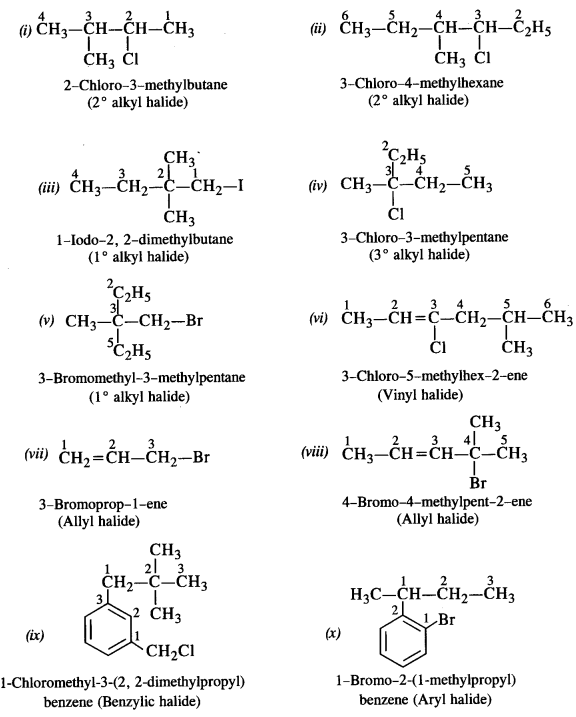
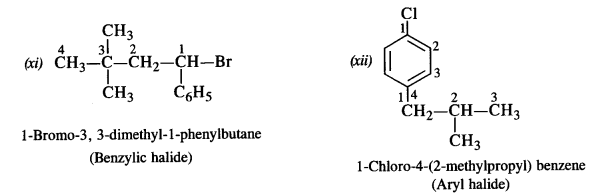
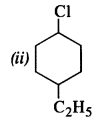
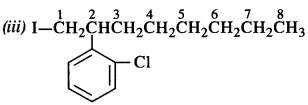
Question 2. Give the IUPAC names of the following compounds :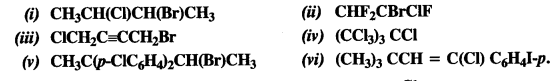
Answer: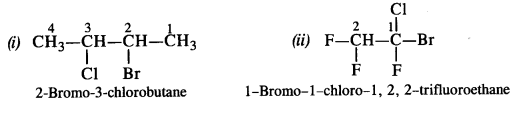
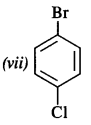
Question 3. Write the structures of the following compounds :
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane.
(iii) 2-(2-Chlorophenyi)-1-iodooctane
(iv) 4-tert. butyl -3-iodooctane
(v) 1, 4-Dibromobut-2-ene
(vi) 1-Bromo-4-sec.butyl-2-methylbenzene.
(vii) p-Bromochlorobenzene
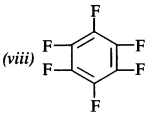
(viii) Perfluorobenzene
Answer: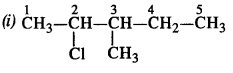
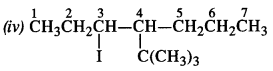
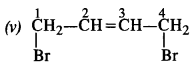
Question 4. Which one of the following has highest dipole moment?
(a) CH2Cl2
(b) CHCl3
(c) CCl4
Answer: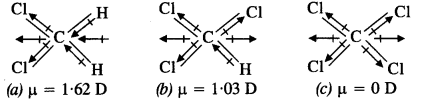
CCl4 is a symmetrical molecule. Therefore, the dipole moments of all four C-Cl bonds cancel each other. Hence its resultant dipole moment is zero.
As shown in the above figure, in CHCl3, the resultant dipole moments of two C-Cl bonds is opposed by the resultant dipole moments of one C-H and one C-Cl bond. Since the resultant of one C-H and one C-Cl bond is smaller than the resultant of the two C-Cl bonds dipole moments, the opposition is to a small extent. As a result CHCl3 has a small net dipole moment.
On the other hand, in case of CH2,Cl2 the resultant of the dipole moments of two C-Cl bonds is strengthened by the resultant of the dipole moments of two C-H bonds. As a result, CH2Cl2 has a higher dipole moment. Hence CH2Cl2 has the highest dipole moments among the three compounds.
Hence, the given compounds can be arranged in the increasing order of their dipole moments as,
CCl4 < CHCl3 < CH2Cl2
Question 5. A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monobromo compound in bright sunlight. Identify the hydrocarbon.
Answer: A hydrocarbon with the molecular formula, C5H10 belongs to the group with a general molecular form CnH2n. therefore, it may either be an alkene or a cycloalkane since hydrocarbon does not react with chlorine in the dark, it cannot be alkene. Further, the hydrocarbon gives a single monochloro compound, C5H9Cl by reacting with chlorine in might sunshine since the formed compound is monochloro one all the C-H bonds should be equivalent. Hence the compound should be a cycloalkane. Hence the compound is C5H10 (cyclopentane).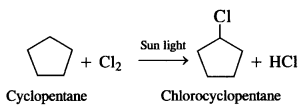
Question 6. Write the isomers of the compound having the formula C4H9Br. (Haryana Board 2013)
Answer: The compound has the following structural isomers.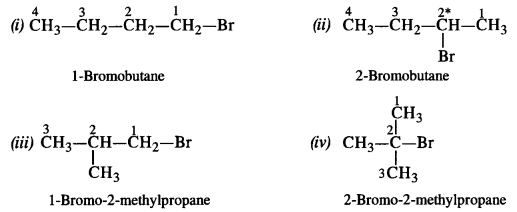
2-Bromobutane has a chiral carbon and it is expected to exhibit optical isomerism.
Question 7. Write equations for the preparation of 1-Iodobutane from :
(a) Butan-1- ol
(b) 1-Chlorobutane
(c) But-1-ene.
Answer: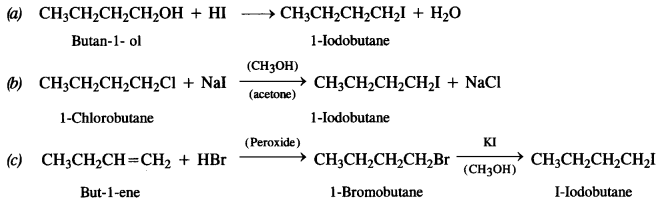
Question 8. What are ambident nucleophiles? Explain with an example.
Answer: Nucleophiles which can attack through two different sites are called ambident nucleophiles. For example, cyanide ion exists as a hybrid of the following two structures. It can attack either![]()
through carbon to form cyanides (or nitriles) or through nitrogen to form isocyanides (or carbyl amines).
Question 9. Which compound in the following pairs will react faster in the Sn2 reaction?
(1) CH3Br or CH3I
(2) (CH3)3CCl or CH3Cl (C.B.S.E. 2008)
Answer:
1. In the SN2 mechanism the reactivity of halides for the same alkyl group increase in order. This happens because as the size increases the halide ion becomes a better leaving group.
R-F << R-Cl < R – Br < R-I
Therefore, CH3I will react faster than CH3Br in SN2 reaction with image 17.
2. The SN2 mechanism involves the attack of the nucleophile at the atom bearing the leaving group. But, in the case (CH3)3 CCl, the attack of the nucleophile at the carbon atom is hindered by the presence of the bulky substituents on that carbon atom bearing-the leaving the group in CH3Cl. Hence CH3Cl reacts faster than (CH3)3 CCl in SN2 reaction with ![]()
Question 10. Predict all the alkenes that would be formed by dehydrohalogenation of following alkyl halides with sodium ethoxide in ethanol.
(i) 1-Bromo-l-metbylcyclohexane
(ii) 2-Chloro-2-methyl butane
(iii) 3-Bromo-2, 2, 3-trimethylpentane.
Answer:
(i) 1-Bromo-l-methylcyclohexane has two β-hydrogen atoms. This will give a mixture of two alkenes as a result of dehydrohalogenation. Since alkene (B) is more substituted according to SaytzefFs rule, it is more stable and will be the major product. The same rule applies to the other alkyl halides also.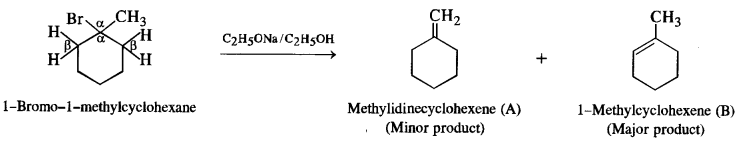
(ii) The compound has two sets of β-hydrogen atoms. Therefore, two elimination products are formed. However, a more substituted alkene is formed in greater proportion as compared to a less substituted alkene.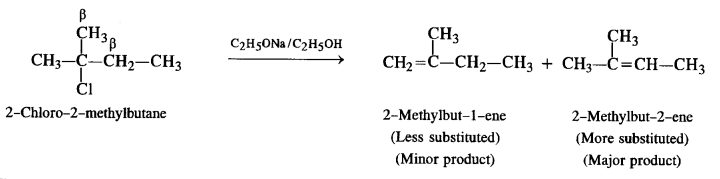
The explanation is similar. More substituted alkene is formed in preference to less substituted alkene.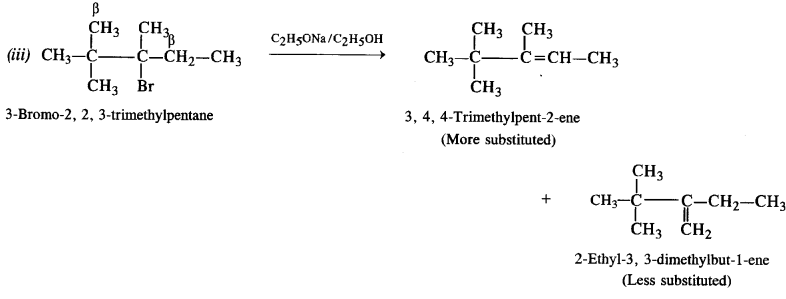
Question 11. How will you bring about the following conversions? (Haryana Board 2011)
(i) Ethanol to but-1-yne
(ii) Ethane to bromoethane
(iii) Propene to 1-nitropropane
(iv) Toluene to benzyl alcohol
(v) Propene to propyne
(vi) Ethanol to ethyl fluoride
(vii) Bromometbane to propanone
(viii) But-1-ene to but-2-ene
(ix) 1-Chiorobutane to n-octane
(x) Benzene to biphenyl
Answer: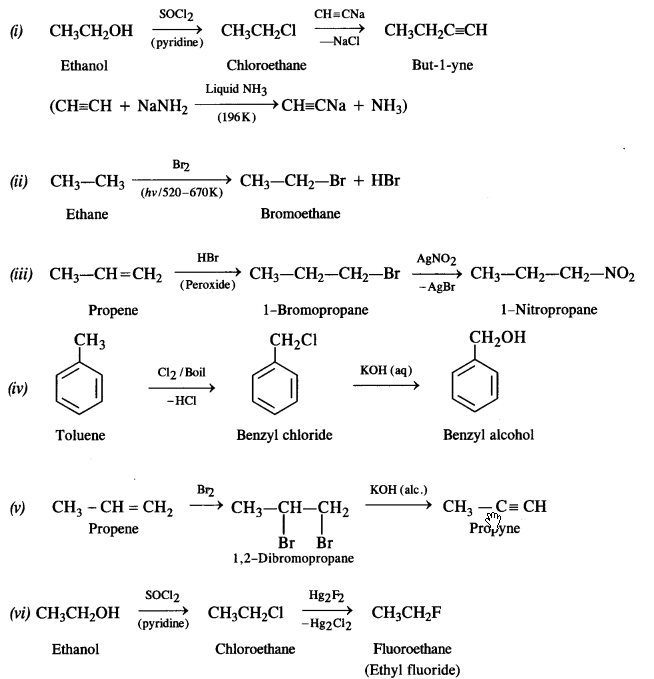
Question 12. Explain why:
(i) Dipole moment of chlorobenzene is lower than that of cyclohexyl chloride (C.B.S.E 2016)
(ii) Alkyl halides though polar, are immiscible with water.
(iii) Grignard reagents should be prepared under anhydrous conditions.
Answer:
The polarity of C- Cl bond in chlorobenzene is less than that of same bond in cyclohexyl chloride because of carbon atom involved in chlorobenzene is more electronegative (greater s-character) as compared to the carbon atom in case of cyclohexyl chloride (lesser s-character). Therefore, the dipole moment of chlorobenzene is less with respect to cyclohexyl chloride.
(ii) In water, H2O molecules are linked to each other by intermolecular hydrogen bonding. Although alkyl halides also contain polar C – X bonds, they cannot break the hydrogen bonding in H20 molecules. This means that there is hardly any scope for the association between molecules of alkyl halides and water. They, therefore, exist as separate layers and are immiscible with each other.
(iii) Grignard reagents (R – Mg – X) should be prepared under anhydrous conditions because these are readily decomposed by water to form alkanes.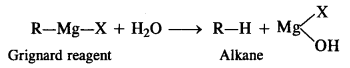
That is why ether used as solvent in the preparation of Grignard reagent is completely anhydrous in nature.
Question 13. Give the uses of freon-12, D.D.T., carbon tetrachloride and iodoform?
Answer:
- Freons are the trade names for the commercially used fluoro chloromethanes with the formula CFxCly (x + y = 4). A few examples are:
CF4 (Freon-14), CF3C1 (Freon-13), CF2Cl2 (Freon-12), CFCl3 (Freon-11)
Out of the various freons mentioned, Freon- 12 is the most common refrigerant. It is prepared by passing hydrogen fluoride
through carbon tetrachionde in the presence of antimony trichioride catalyst.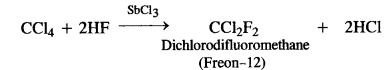
in addition to their use as refrigerants in place of highly toxic liquid sulphur dioxide (SO2) and ammonia (NH3), large amount of CFCs are also used in the manufacture of disposable foam products such as cups and plates, as aerosol propellants in spray cans and as solvents to clean freshly soldered electronic circuit boards. - D.D.T. is the abbreviated form of p, p’-dichlorodiphenyltrichloroethane and its actual IUPAC naine has been given above. It is
prepared by heating chiorobenzene with chlorai (trichioroacetaldehyde) in the presence of conc. H2S04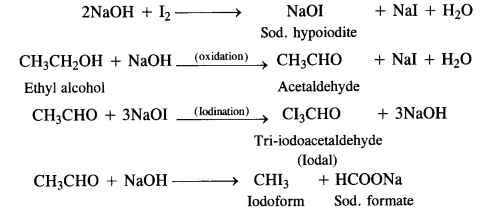
- Carbon tetrachloride (CC14) is also a colourless oily liquid just like chloroform. It is completely immiscible with water but
dissolves in organic solvents.
Carbon tetrachloride is a very useful solvent for oils, fats, resins etc. Ills used as a cleansing agent both in industry and in home because it can easily dissolve grease and other organic matter. But it mainly finds application for the manufacture of refrigerants, propellants for aerosol cans and some pharmaceuticals. - lodoform is a yellow crystalline solid with a characteristic unpleasant smell. It is insoluble in water but dissolves in alcohol, ether and other organic solvents.
lodoform can be prepared in the laboratory by treating ethyl alcohol or acetone with sodium hydroxide and iodine. The reaction is known as haloform or iodoform reaction. - Physiological effects: lodoform is used as an antiseptic, particularly for dressing wounds. Actually, on coming in contact with skin (organic mater) it decomposes and slowly loses iodine which accounts for the antiseptic properties of iodoform.
Question 14. Write the structures of the major products in each of the following reactions :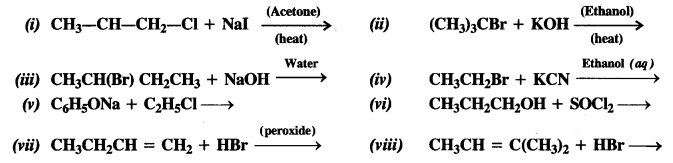
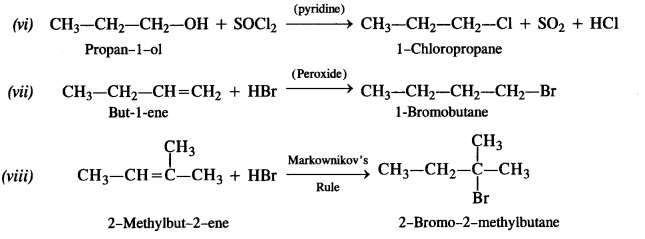
Answer: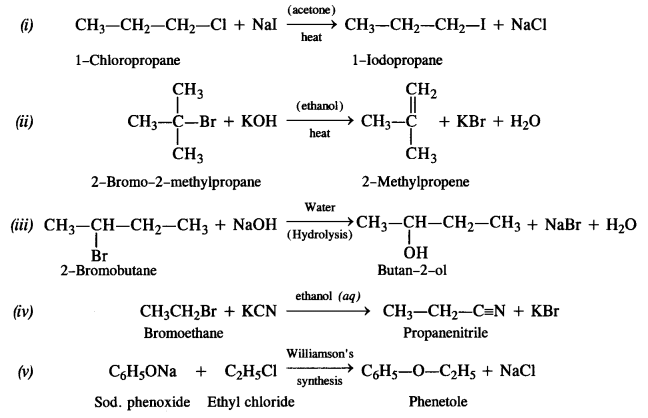
Question 15. Explain the following reaction : (C.B.S.E. Delhi 2009 Comptt.)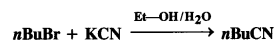
Answer: KCN is a resonance hybrid of two contributing structures :![]()
This shows that the cyanide ion is an ambident nucleophile and the nucleophile attack is possible either through carbon atom or nitrogen atom resulting in cyanides and isocyanides respectively. In this case, in the presence of polar solvent, KCN readily ionises to furnish ions. The nucleophile attack takes place predominantly through a carbon atom and not through nitrogen atom as C- C bond is more stable than C -N bond.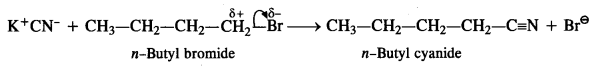
Question 16. Arrange the compounds of each set in order of decreasing reactivity towards (SN²) displacement:
(a) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
(b) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 2-Bromo-3-methylbutane
(c) 1-Bromobutane, l-Bromo-2, 2-dimethylpropane, l-Bromo-2-methylbutane, l-Bromo-3-methylbutane. (C.B.S.E. Outside Delhi 2011)
Answer: The reactivity of a particular haloalkane towards SN2 reaction is inversely proportional to the steric hindrance around the carbon atom involved in C – X bond. More the steric hindrance, lesser will be the reactivity. In the light of this, the decreasing order of reactivity in all the three cases is as follows :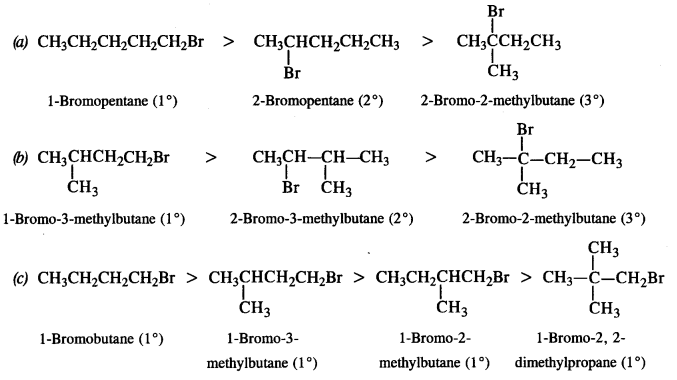
Question 17. Out of C6H5CH2Cl and C6H5CH(C1)C6H5 which is more easily hydrolysed by aqueous KOH?
Answer: The compound C6H5CH2Cl is a primary aralkyl halide while C6H5CH(Cl)C6H5 is secondary in nature. The hydrolysis of both these compounds with aqueous KOH (polar) is likely to proceed by SN¹ mechanism due to the following reasons.
(a) The carbocations formed in both the cases as a result of ionisation are resonance stabilised due to the presence of phenyl groups at the a-position(s).
(b) As water is a polar solvent, it is expected to favour ionisation of the two halogen-substituted compounds leading to SN¹ mechanism.
The carbocations that are formed as a result of ionisation in the slow steps are shown :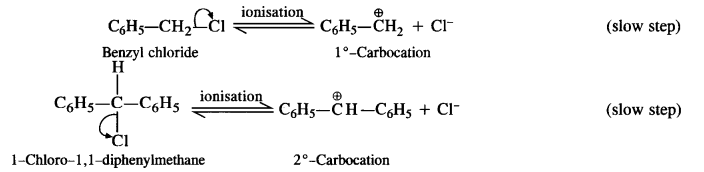
The ease of hydrolysis depends upon the relative stability of the carbocation/s that are formed in two cases. The secondary carbocation is more stable since the positive charge on the carbocation is delocalised on two phenyl groups that are present at the a-positions. On the other hand, there is only one phenyl group in primary carbocation available for charge delocalisation.
Thus, we may conclude that C6H5CHClC6H5 is more easily hydrolysed by aqueous KOH as compared to C6H5CH2Cl.
Question 18. p-Dichlorobenzene has higher m.p. and lower solubility than those of o-and m-isomers. Discuss.
Answer: p-dichlorobenzene has a higher melting point than its o-isomer due to the symmetry of the p-isomer that fits in the crystal lattice better than the o- or m- isomer. Therefore, it has stronger intermolecular forces of attraction than o- and m- isomers, and thus greater energy are required to break crystal lattice to melt or dissolve the p-isomer than the corresponding o- and m- isomers. In other words, the melting point of the p-isomer is higher and its solubility is lower than corresponding m- and o- isomers.
Question 19. How the following conversions can be carried out?
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenyl ethanoic acid
(vii) Ethanol to propane nitrite
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3, 4 – dimethyl hexane
(x) 2-Methylpropene to 2-chloro-2-methylpropane
(xi) Ethyl chloride to propanoic acid
(xii) But-l-ene to n-butyl iodide
(xiii) 2-Chloropropane to propan-l-ol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
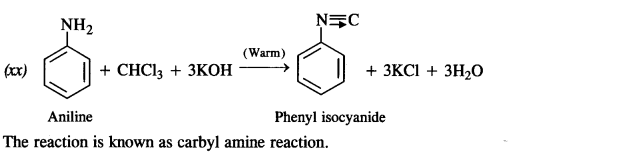
(xx) Aniline to phenyl isocyanide.
Answer: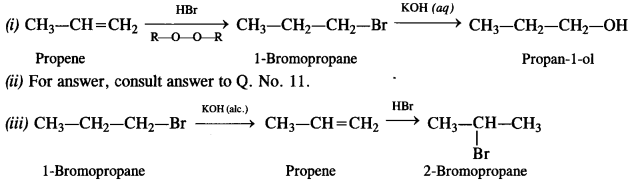
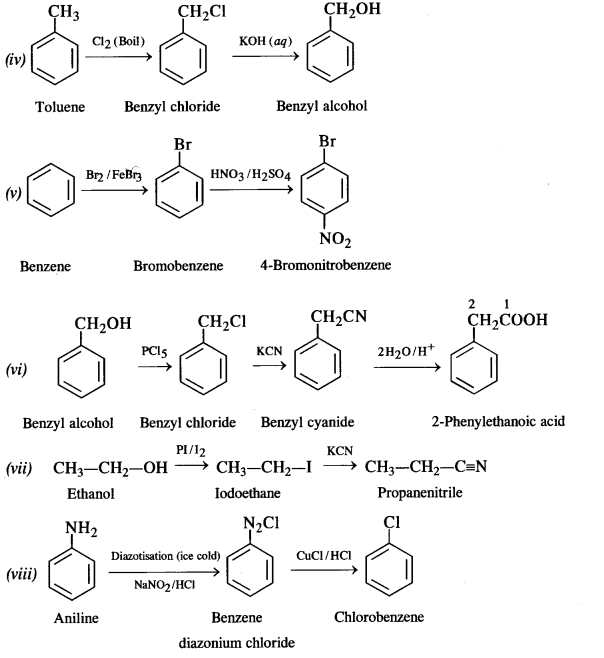
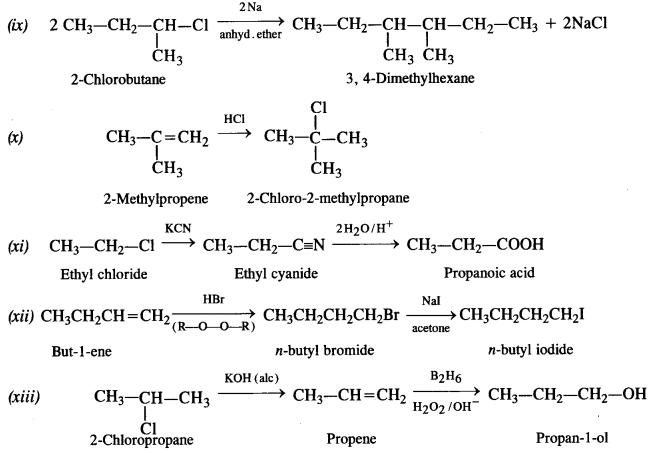
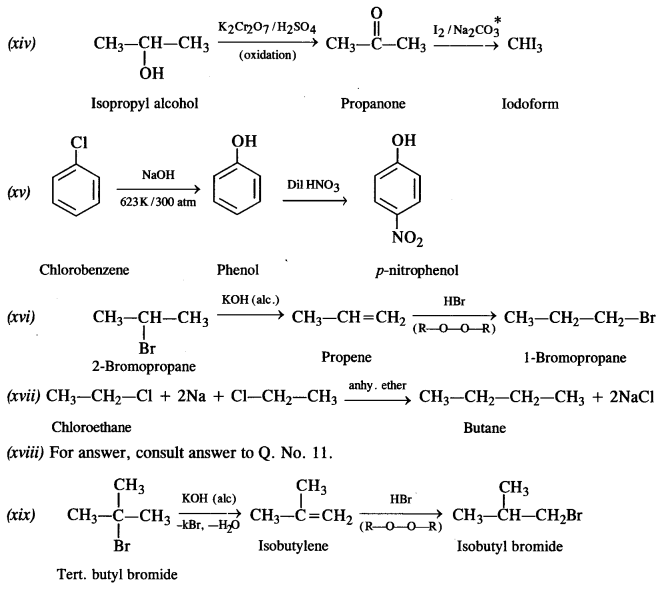
Question 20. The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Answer: If aqueous solution, KOH is almost completely ionized to give OH– ions which being a strong nucleophile brings about a substitution reaction on alkyl halides to form alcohols. Further in the aqueous solution, OH– ions are highly solvated (hydrated). This solvation reduces the basic character of OH– ions which, therefore, fails to abstract a hydrogen from the P-carbon of the alkyl chloride to form alkenes. In contrast, an alcoholic solution of KOH contains alkoxide (RO–) ion which being a much stronger base than OH– ions preferentially eliminates a molecule of HCl from an alkyl chloride to form alkenes.
Question 21. Primary alkyl halide (a) C4H9Br was reacted with alcoholic KOH to give compound (b). Compound (b) was reacted with HBr to give (c) which was an isomer of (a). When (a) was reacted with sodium metal, it gave a compound (d) C8H18, that was different than the compound when n-butyl bromide was reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Answer: The two primary alkyl bromides are possible from the molecular formula (a) C4H9Br. These are: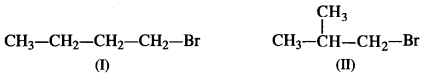
According to the available information, the isomer (I) does not represent the correct compound because this on reacting with sodium metal (Wurtz reaction) will give n-octane. (C8H18) which is not actually formed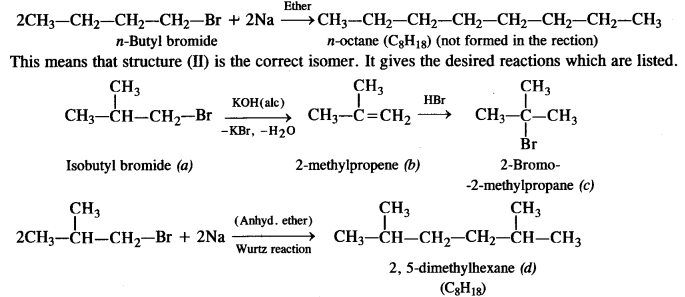
Question 22. What happens when
(i) n-butyl chloride is treated with alcoholic KOH,
(ii) bromobenzene is treated with Mg in the presence of dry ether,
(iii) chlorobenzene is subjected to hydrolysis,
(iv) ethyl chloride is treated with (aq.) KOH,
(v) methyl bromide is treated with sodium in the presence of dry ether,
(vi) methyl chloride is treated with KCN?
Answer:
(i) But-l-ene is formed as the product as a result of dehydrohalogenation.![]()
(ii) Phenyl magnesium bromide (Grignard reagent) is formed as a result of the reaction.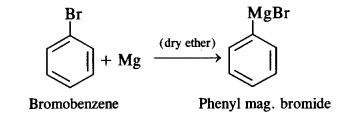
(iii) Chlorobenzene will not get hydrolysed on boiling with NaOH. No product will be formed.
(iv) Ethyl alcohol is formed as the product![]()
(v) Ethane is formed as a result of Wurtz reaction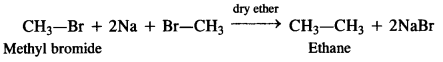
(vi) Methyl cyanide is formed.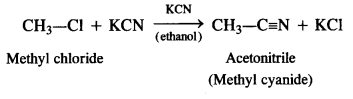














thanks sir for the solutions of this chapter
ReplyDelete