NCERT Solutions for Class-11 Chemistry Chapter-14 Environmental Chemistry
NCERT IN-TEXT QUESTIONS
Question 1. Define environmental chemistry.
Answer: Environmental chemistry is the branch of science which deals with the chemical changes in the environment. It includes our surroundings such as air, water, soil, forests, sunlight etc.
Question 2. Explain tropospheric pollution in 100 words.
Answer:
Tropospheric Pollution: Tropospheric pollution is caused by both inorganic and organic gases. Gases like oxides of nitrogen, oxides of sulphur, oxides of carbon, H2S, HCN, HC1 etc. constitute inorganic pollutants.
Organic pollutants include mercaptants, hydrocarbons, formaldehyde, alcohol, certain organic acids chlorinated hydrocarbon etc.
Some of these are released as such in the atmosphere and are known as primary pollutants. Some others are formed in the atmosphere as a result of chemical reactions.
These are called secondary pollutants. A few examples are : Ozone, chlorofluorocarbons (CFCs), formaldehyde, acrolein, methyl isocyanate etc. Let us briefly study some of the tropospheric pollutants.
Answer:
Carbon monoxide: (CO) when inhaled reacts with haemoglobin (Hb) to form complex carboxy haemoglobin (CoHb). The compound formed is not in a position to transport the inhaled oxygen to the various parts of the body. On the other hand, the presence of carbon dioxide can lead only to green house effect causing global warming.
Carbon dioxide: Carbon dioxide, is present in air in about 0.03% by volume. It is being constantly released into the atmosphere by the combustion of fossil fuels such as coal and oil for energy.
In addition to this, volcanic erruptions and decomposition of lime stone release carbon dioxide in the atmosphere. However, it is also taken away from the atmosphere regularly by green plants and forests because the plants need carbon dioxide for photosynthesis,
Thus carbon dioxide cycle is working round the clock which maintains its percentage in the atmosphere. However, with the deforestation that has taken place, there is an increased build up of the gas in the atmosphere.
Question 4. Which gases are responsible for green house effect ? Name them.
Answer: The green house effect is caused by the following gases which are capable of trapping heat energy.
(i) carbon dioxide
(ii) methane
(iii) ozone
(iv) chlorofluorocarbon compounds (CFC’s)
(v) water vapours.
Question 5. Statues and monuments in India are affected by acid rain. How ?
Answer: The statues and monuments are mainly made from marble which is chemically calcium carbonate (CaCO3). Acid rain has vapours of sulphuric acid dissolved in it. When it comes in contact with the various statues or monuments, the acid reacts chemically with calcium carbonate. As a result of the chemical reaction, the material of the statues are slowly eaten up.
It is also called corrosion. Thus, acid rain is a threat to our precious historical monuments and statues.
CaCO3 + H2SO4 → CaSO4 + H2O + CO2
Question 6. What are smogs ? How are classical and photochemical smogs different ?
Answer: Air pollution is commonly caused in big and industrial cities in the form of smog which is quite often termed as smoke- smog. This may be either classical or photochemical in nature.
Question 7. Write chemical reactions involved during the formation of photochemical smog.
Answer: 
Question 8. What are the harmful effects of the photochemical smog ? How can they be controlled ?
Answer: Harmful Effects of Photochemical Smog.
The main constituents of photochemical smog are ozone, oxides of nitrogen, acrolein, formaldehyde and peroxyacetylnitrate (PAN). These are responsible for the harmful effects. A few out of these are listed.
(i) Ozone and nitric oxide cause irritation in nose as well as in throat. Their high concentration usually causes headache and chest pain.
(ii) The gases which constitute photochemical smog, usually cause dryness of throat, cough and are responsible for breathing problems.
(iii) Photochemical smog causes substantial damage to plant life.
(iv) It also results in corrosion of metals, building materials, rubber and painted surface etc.
How to control Photochemical Smog: Following measures can check the pollution caused by photochemical smog to some extent.
(i) Use of catalytic converters in the engines of automobiles will check the release of both N02 and certain hydrocarbons known as primary precursors. This will automatically check the formation of secondary precursors such as ozone and PAN which are harmful.
(ii) Certain plants like pinus, pyrus, vitis quercus etc. are capable of causing the metabolism of the oxides of nitrogen which are quite harmful. Their plantation will definitely help in checking the spread of these gases in the atmosphere.
Question 9. What are the reactions involved for ozone layer depletioh in stratosphere ?
Answer: Chlorofluorocarbons such as freon etc. present in the stratosphere are involved in the chemical reaction with ozone. These are of free radical nature and carried in the presence of U. V. radiations.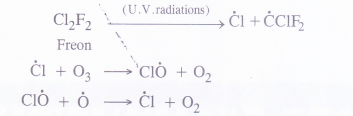
Since ozone takes part in the chemical reaction, there is a gradual depletion of ozone layer which is taking place.
Question 10. What do you understand by ozone hole ? What are its consequences ?
Answer: Ozone hole implies destruction of the ozone layer by the harmful ultraviolet (UV) radiations. The depletion will virtually result in creating some sort of holes in the blanket of ozone which surrounds us. As a result, the harmful radiations will cause skin cancer, loss of sight and will also affect our immune stystem.
The depletion of ozone layer also called ozone hole was noticed in the year 1980 by the scientists working in Antarctica region in the South Pole. Special conditions prevailing in the region were responsible for the depletion of ozone. During summer, nitrogen dioxide and methane react with chlorine monoxide and chlorine atoms (as free radicals)
CI\(\dot { O } \) (g) + NO2(g) → ClONO2(?)
Chlorine nitrate
\(\dot { C } \)l (g)+ CH4(g) → \(\dot { C } \)H3 (g) + UCl(g)
Under the conditions, \(\dot { C } \)l are not in a position to react with ozone and thus, the depletion of ozone layer is checked. During winter, special types of clouds known as polar stratospheric clouds get formed over Antarctica region. They provide a surface over which chlorine nitrate react with water vapours and also with hydrogen chloride gas.
ClONO2(g) + H2O (g) → HOCl(g) + HNO3(g)
ClONO2(g) + HCl(g) → Cl2(g) + HNO2(g)
During spring season, sunlight returns and its warmth cleaves both HOCl and Cl2 to form free radicals. In other words, they undergo photolysis.![]()
Cl2(g)→ 2\(\dot { C } \)l(g)
The \(\dot { C } \)l flee radicals initiate chain reaction with ozone resulting in the depletion of ozone layer or forming ozone hole.
Question 11. What are the major causes of water pollution ? Explain.
Answer:
(i) These are decomposed by aerobic bacteria into carbon dioxide, nitrates, sulphates, phosphates etc. but they take up dissolved oxygen from water.
(ii) As a result, the oxygen contents in water decrease considerably.
(iii) This causes the death of aquatic animals particularly the fish.
(iv) It may be noted that anaerobic bacteria do not need oxygen from the decomposition of the organic matter. However, some toxic gases like hydrogen sulphide, ammonia, phosphine, methane etc. are produced.
(v) These are quite often noticed in the sewage wastes.
(vi) We know that the presence of oxygen in water is quite essential for the aquatic life.
(vii) The source of oxygen in water is natural aeration or photosynthesis carried by water plants during day time in the presence of sun light.
The quantity of the oxygen consuming wastes in water can be determined in terms pf biological oxygen demand (B.O.D.).
It may be defined as: the amount of oxygen in milligrams dissolved in water needed to break down the organic matter present in one litre of water for five days at 20°C.
2. Industrial wastes. The compounds of lead, mercury, cadmium, nickel, cobalt, zinc etc. which are the products of chemical reactions carried in the industrial units also pollute water to a large extent and are responsible for many diseases.
Mercury leads to minamata disease,’and lead poisoning leads to various types of deformaties. In addition to this, these chemical substances become apart of soil. They harmfully affect the plant growth and soil biota.
Both ground water and water bodies are polluted due to the chemical reactions known as leaching.
3. Fertilizers. These are the chemical substances which are added to the soil to provide the essential minerals containing N, P, S etc.
The common fertilizers are calcium ammonium nitrate, urea, triple super phosphate, potassium sulphate, potassium nitrate etc. However, a certain part of these fertilizers react with water chemically (known as leaching) and pollute the underground water.
When this water is used for drinking purposes containing potassium nitrate in particular, it harms the respiratory system. Moreover the presence of extra minerals in water is also harmful to many crops.
Question 12. Have you ever observed any water pollution in your area ? What measures would you suggest to control the same.
Answer:
Control of Water Pollution: We have seen that the two major sources of water pollution are : sewage and industrial wastes. They should be removed from water before it is put to use.
Treatment of Sewage. Following measures must be taken to check pollution by sewage.
(i) Sewage must be churned by machines so that the large pieces may break into smaller ones and may get mixed thoroughly. The churned sewage is passed into a tank with a gentle slope. Heavier particles settle and the water flowing down is relatively pure.
(ii) Water must be sterilised with the help of chlorination. It kills microbes of sewage fungus as well as some pathogens, spores or cytes. Chlorination is very essential particularly in rainy season.
(iii) Treatment of water with alum, lime etc. also helps in its purification.
Treatment of industrial waste. The treatment of industrial waste depends upon the nature of the pollutants present. In order to ascertain it, the pH of the medium is first determined and the waste is then neutralised with the help of suitable acids or alkalies.
The chemical substances present in the industrial waste products dissolved in water can be precipitated by suitable chemical reactions and removed later on from water. Quite recently, photocatalysis and ion-exchangers have been developed for the treatment of industrial wastes.
Question 13. What do you understand by biochemical oxygen demand (B.O.D.) ?
Answer: It may be defined as:
the amouitt of oxygen in milligrams dissolved in water needed to break down the organic matter present in one litre of water fob five days at 20° C.
Pure water contains B.O.D. upto 3 ppm. In case, this level is more, it will suggest the presence of organic waste in water which consume oxygen.
Answer: Faulty Agricultural Practices. In the present era, the major thrust is to get more yield of the crop and on intensive farming. This employs the use of a lot of fertilizers, pesticides, weedicides etc. All of them are chemical substances and from the soil they pass to the ground water and are harmful to the aquatic animals. Moreover, water develops foul smell, bad taste and also acquires some brown colour.
Control of Soil Pollution: In order to control soil pollution, the following measures are necessary:
(i) Use of manures. Manure is a semi-decayed organic matter which is added to the soil to maintain its fertility. These are mostly prepared from animal dung and other farm refuse. These are much better than the commonly used fertilizers. •
(ii) Use of bio-fertilizers. These are organisms which are inoculated in order to bring about nutrient enrichment of the soil e.g., nitrogen fixing bacteria and blue-green algae.
(iii) Proper sewerage system. A proper sewerage system must be employed and sewerage recycling plants must be installed in all towns and cities.
(iv) Salvage and recycling. Rag pickers remove a large number of waste articles such as paper, polythene, card board, rags, empty bottles and metallic articles. These are subjected to recycling and this helps in checking soil pollution.
Question 15. What are pesticides and herbicides ? Explain giving examples.
Answer:
Pollution by Pesticides: We have so far discussed the pollution resulting from air, water and soil. In addition to these, pesticides are the major pollutants. These are the chemical substances which contaminate our food as well as drinking water. In fact, their major role is to kill or block the reproductive processes in organism which are not needed and, thus, save the soil from pests. These have been classified in three types.
Insecticides: Insecticides are the chemical substances which destroy the bacterias causing malaria and yellow fever. Moreover, they also protect the crops from various insects.
The best known insecticide is D.D.T. (dichlorodiphenyltrichloroethane). Since it is an organo chloro coippound, the chlorine acts as a toxic to insects. It also does not dissolve in water and there is no danger of causing water pollution.
However, major disadvantage is because of its non-biodegradable nature. It gets accumulated in the environment and has many harmful effects. It is no longer being used and is replaced by a better insecticides like BHC.
Question 16. What do you understand by green chemistry ? How will it help in decreasing environmental pollution ?
Answer: We have studied that the major cause of environment pollution is the release of toxic chemicals which are formed as a result of the processes and reactions carried at various levels. In other words, chemists are mainly responsible for polluting the atmosphere although they manufacture products that are source of our comfort. This has forced them to change their outbook. Since 1990, a new concept called Green Chemistry has been introduced.
(ii) No doubt this is altogether a new field but some success has been achieved. Efforts have been made to carry the reactions in the presence of ultraviolet sunlight (known as photochemistry) and with sound waves (called sonochemistry).
(iii) Microwaves have been used to carry reactions which neither need toxic solvents nor release such vapours into the atmosphere.
(iv) Automobile engines have been fitted with catalytic converters which prevent the release of the vapours of hydrocarbons and oxides of nitrogen into the atmosphere.
(v) These are the real culprits since they form poisonous substances such as formaldehyde, acrolein and peroxyacetyl nitrate.
(vi) Carbon dioxide has replaced of chlorofluorocarbons as blowing agents in the manufacture of polystyrene foam sheets.
(vii) This has checked the release of chemicals into the environment which cause depletion of ozone layer.
(viii) Similarly, some enzymes have been employed as biocatalysts in the manufacture of certain antibiotics such as ampicillin and amoxycillin.
(a) Dry cleaning of clothes. A commonly used dry cleaning solvent is tetrachloroethene (Cl2C = CCl2). It pollutes water and is also carcinogenic. This has been replaced by some other detergents which contain liquid carbon dioxide. These do not pollute water and give better results. These days, hydrogen peroxide is being used in the laundries for removing stains from clothes.
(b) Bleaching of paper. Chlorine gas was used earlier for bleaching of paper. It releases poisonous fumes in the atmosphere. At present, hydrogen peroxide is also used for this purpose.
(c) Synthesis of chemicals. Ionic catalysts in the form of Pd2+ and Cu2+ salts have been employed for the preparation of acetaldehyde (ethanal) from etIrene by carrying oxidation with oxygen. The yield of ethanal is excellent (about 90%)
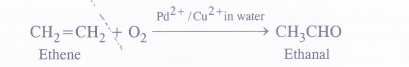
This is just the beginning and the results are encouraging. We are sure, our scientists particularly the chemists will be successful in developing new techniques as well as chemicals to minimise pollution. All countries are giving importance to the study of biotechnology which has a major role in checking pollution. It is the duty of every individual to keep the environment free from pollution.
Question 17. What would happen if green house gases were totally missing in earth’s atmosphere ? Discuss.
Answer: The solar energy which is radiated back from the earth surface is absorbed by different green house gases that we have listed. As a consequence of it, the atmosphere around the surface of the earth becomes warm. This helps in the growth of vegetation and also supports life. In the absence of this effect, there will be no life of both plant and animal on the surface of the earth.
Question 18. A large number of fish are suddenly found floating dead on a lake. There is no evidence of toxic dumping but you can find an abundance of phytoplankton. Suggest a reason for the fish kill.
Answer: Phytoplankton growth occurs in water because of the presence of organic matter like leaves, grass, trash etc. in water. It is likely to consume a lot of oxygen dissolved in water which is of course very much essential for the life of sea animals particularly fish.
If the level of dissolved oxygen in water is below 6 ppm, this means that the oxygen is not sufficiently available to the variety of fish living in water. They are likely to perish or die. This might have happened in this particular case.
Question 19. How can domestic waste be used as manure ?
Answer: Domestic waste consists of both biodegradable and non-biodegradable components. The latter consisting of plastic, glass, metal scrap etc. is separated from it. The biodegradable portion which consists of organic matter can be converted into manures by suitable methods.
Question 20. For your agriculture field or garden, you have developed a compost producing pit. Discuss the process in the light of bad odour, flies and recycling of wastes for a good produce.
Answer: For the healthy growth of plants and grass in the garden, compost is periodically required. Compost producing pit is usually created nearly provided space is available. Difficulties do arise in urban areas due to paucity of space.
The pits generally give foul smell and flies roam about. This is very bad for health. In order to check it, the pits must be properly covered.
The waste materials such as glass articles, plastic bags, old newspapers etc. must be handed over to the vendors regularly. These are ultimately sent to recycling plants without creating pollution problems.

0 comment
Post a Comment