NCERT Solutions for Class-12 Chemistry Chapter-11 Alcohols, Phenols and Ethers
NCERT INTEXT QUESTIONS
Question 1. Classify the following into primary, secondary, and tertiary alcohols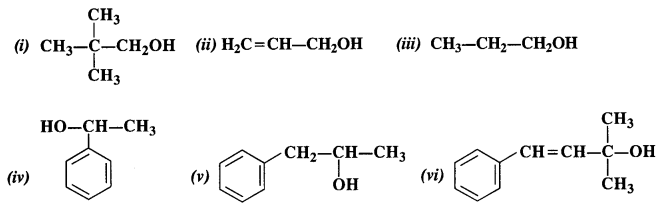
Answer:
(i) Primary alcohol
(ii) Primary alcohol
(iii) Primary alcohol
(iv) Secondary alcohol
(v) Secondary alcohol
(vi) Tertiary alcohol
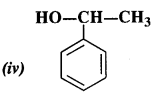
Question 2. Identify allylic alcohol in the above examples.
Answer: allylic alcohols: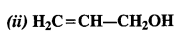
Question 3. Give the IUPAC names of the following compounds : (C.B.S.E 2008)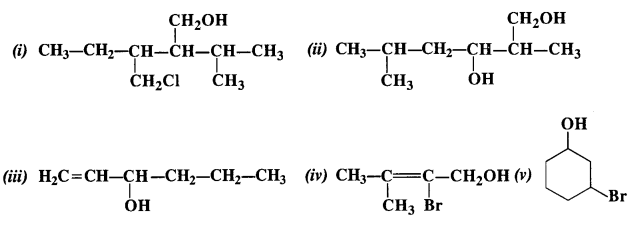
Answer: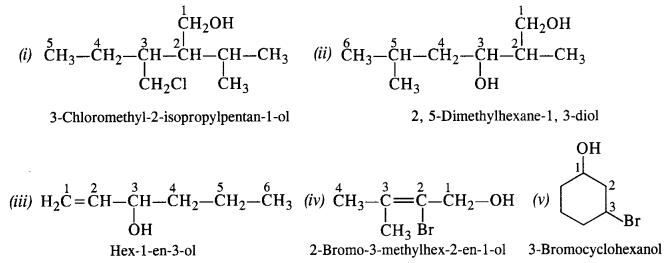
Question 4. Show how the following alcohols can be prepared by the action of suitable Grignard reagent on methanal ?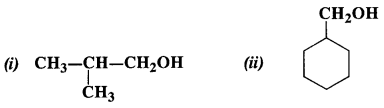
Answer:
(i) The structure of alcohol suggests that the Grignard reagent that reacts with methanal is isopropyl magnesium halide.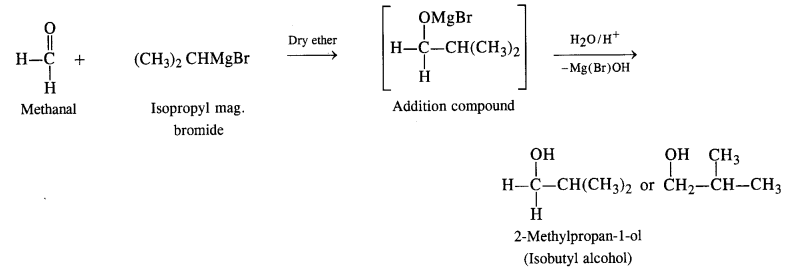
(ii) The structure of alcohol suggests that the Grignard reagent that reacts with methanal is cyclohexyl magnesium halide.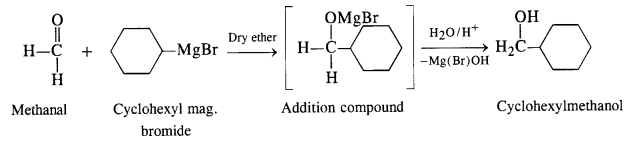
Question 5. Write the structures of the products of the following reactions :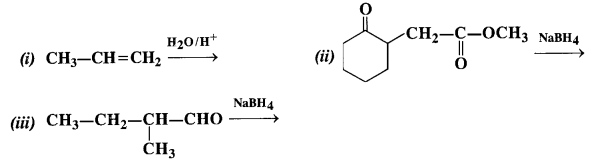
Answer:
(i) The acidic hydration of propene gives propan-2-ol (isopropyl alcohol)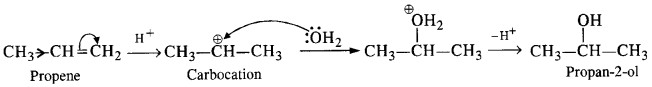
(ii) NaBH4 is a weak reducing agent. It brings about the reduction of the ketonic group present in cyclohexane ring to secondary alcoholic group. However, it does not affect ester group.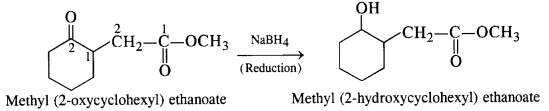
(iii) NaBH4 reduces aldehydic group to a primary alcoholic group.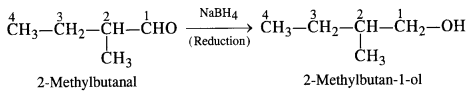
Question 6. Give structures of the products you would expect when each of the following alcohols reacts with (a) HCl/ZnCl2 (b) HBr (c) SOCl2 :
(i) Butan-l-ol
(ii) 2-Methylbutan-2-ol
Answer:
(i) Reactions of Butan-1-ol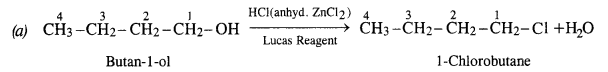
The reaction of butan-l-ol (primary alcohol) can take place only upon heating. At room temperature, the reaction does not occur.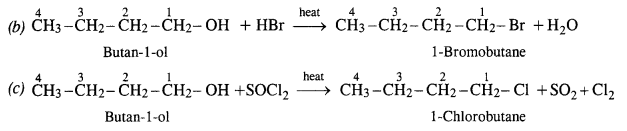
(ii) Reactions of 2-Methylbutan-2-ol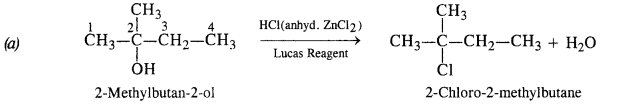
The alcohol being tertiary in nature reacts immediately with Lucas Reagent at room temperature.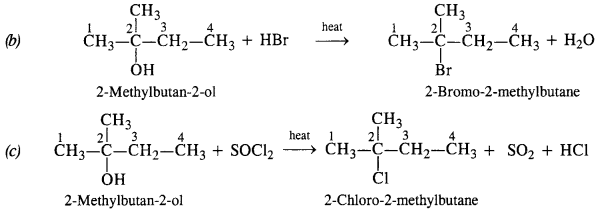
Question 7. Predict the major product of add catalysed dehydration of :
(i) 1-Methylcyclohexanol
(ii) Butan-1-ol
Answer:
(i) Acid catalysed dehydration of 1-methylcyclohexanol can give two products. However, 1-methylcyclohexene will be preferably formed according to Satyzeff s rule since it is more substituted.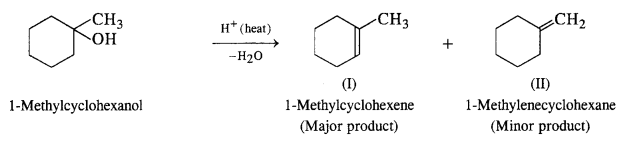
(ii) Butan-1-o1 upon acid dehydration will give but-2-ene as the major product along with but-l-ene as the minor product. Actually, primary carbocation formed can either lose a H+ to form but-1-ene or may undergo rearrangement and shift to secondary carbocation, which is more stable. The latter then forms but-2-ene by losing a H+.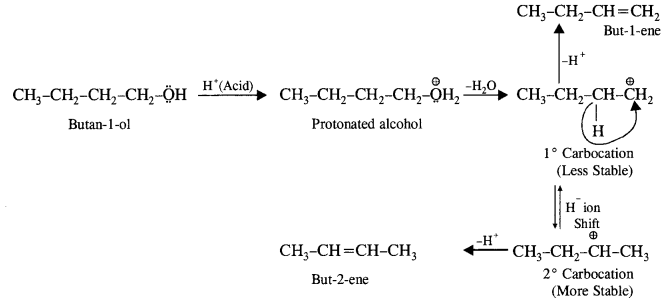
Question 8. Ortho and para nitrophenols are more acidic than phenol. Draw the resonating structures of the corresponding phenoxide ions.
Answer: We know that the nitrophenols are more acidic than phenol. Their acidic strength can be compared in terms of the relative stabilities of the corresponding phenoxide ions based on resonance. For example,
(i) Phenoxide ion :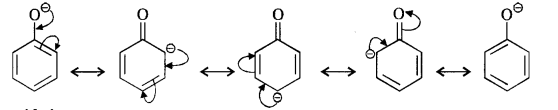
(ii) p-Nitrophenoxide ion :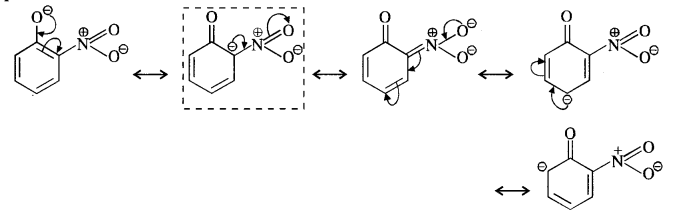
(iii) p-Nitrophenoxide ion :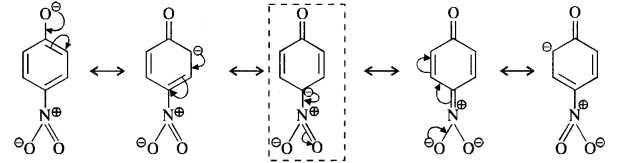
In case of nitrosubstituted phenoxides, the contributing structures that are enclosed in boxes have negative charge on the carbon atom to which die electron withdrawing nitro group is attached. They therefore, contribute more towards the acidic character than the rest of the contributing structures. Consequently, both ortho and para nitrophenol are stronger acids than phenol.
Question 9. Write the equations involved in the following reactions :
(i) Reimer Tiemann Reaction
(ii) Kolbe’s Reaction.
Answer:
(i) In this reaction phenol is heated with chloroform alongwith aqueous NaOH (or KOH) to about 340 K. This is followed by acidification with dilute HCl when 2 – hydroxyhenzaldehyde (salicylaldehyde) is formed as the major product.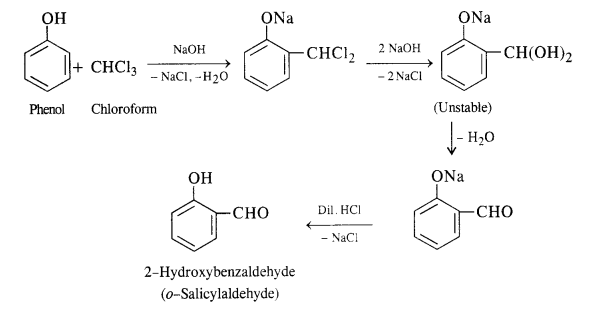
A small amount of para isomer is also formed in the reaction. In case, chLoroform is replaced by carbon tetrachioride, then 2—hydroxybenzoic acid (salicylic acid) is formed as the main product.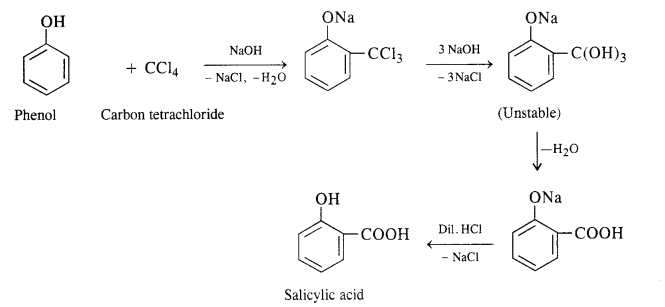
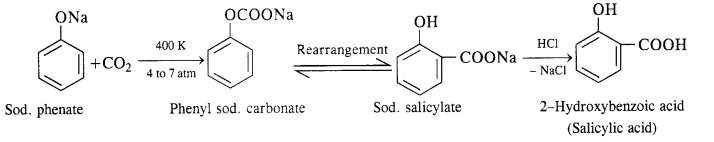
(ii) In this reaction, CO2 gas is passed through sodium phenate at 400 K under a pressure of 4 to 7 atmospheres. This is followed by acidification with dilute HCI when salicylic acid is formed. This method is commonly used for the commercial preparation of saucy lic acid.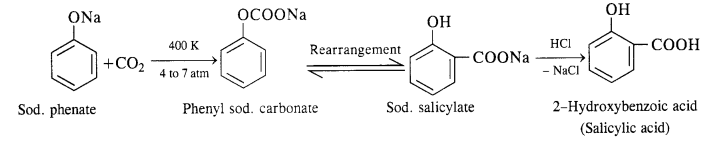
Question 10. Write the reactions of Williamson’s synthesis of 2-ethoxy-3-methoxypentane starting from ethanol and 3-methylpentan- 2-ol.
Answer: In the Williamson’s synthesis, the reactants are alkyl halide and sodium salt of an alcohol. In order to avoid the formation of alkene during the reaction, the alkyl halide should be primary while sodium salt must be of branched chain alcohol. In the present case, alkyl halide must be derived from ethanol upon heating with halogen acid (e.g HBr).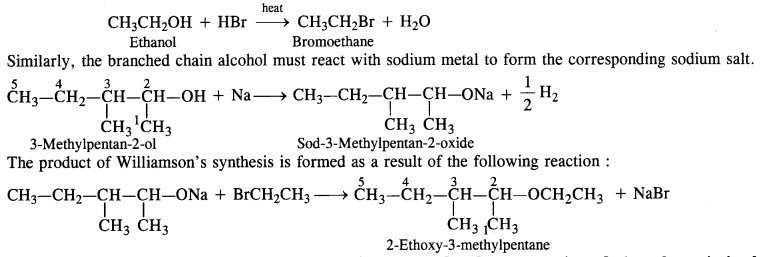
Question 11. Which of the following is an appropriate set of reactants for the preparation of l-methoxy-4-nitrobenzene and why ?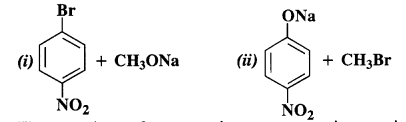
Answer: The second set of reactants is more appropriate to give the products i.e., l-methoxy-4-nitrobenzene. In the first set, cleavage of C – Br bond is involved. It is rather difficult since the carbon atom is sp2 hybridised and the bond has partial double bond character as well. The product is formed as a result of Williamson’s synthesis.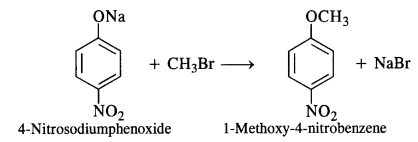
Question 12. Predict the products of the following reactions :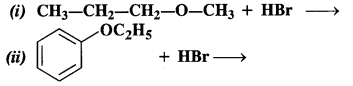
Answer:
(i) The reaction involves the cleavage of C – 0 bond. The Br atom of HBr is to combine with the smaller alkyl group to give the following products.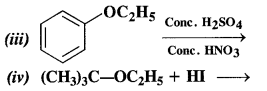
(ii) This reaction also proceeds in the same manner. The Br atom of HBr is expected to combine with ethyl group (smaller in size) and not with phenyl group (bigger in size).![]()
(iii) Nitrating mixture brings about the nitration of benzene ring. The ethoxy group (OC2H5) is an activating group and increases the electron density at the ortho and para positions due to +M effect. As a result, a mixture of o-nitro and p-nitro derivatives is formed. Out of these, the p-isomer is in excess since there is less steric hindrance due to OC2H5 group at the para position than at the ortho position in the ring.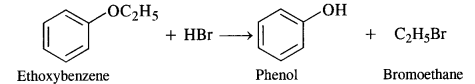
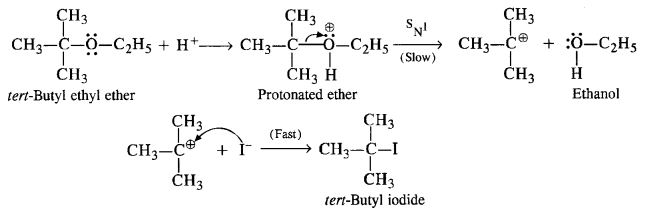
(iv) In this reaction, the ether is initially protonated by H+ ion of the acid HI. to accomodate I– ion (nucleophile). The reaction follows Sn1 mechanism.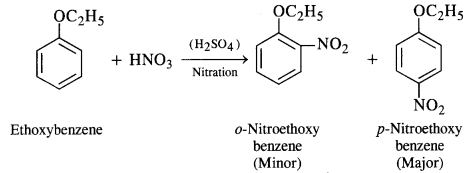
NCERT EXERCISE
Question 1. Write IUPAC names of the following compounds :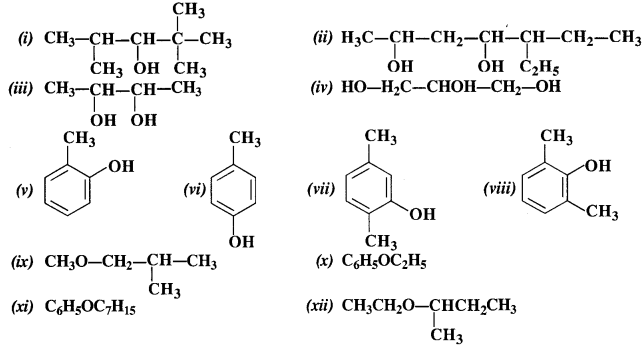
Answer:
(i) 2, 2, 4 – Trimethylpentan-3-ol
(ii) 5 – Ethylheptan-2, 4-diol
(iii) Butane – 2, 3, diol
(iv) Propane – 1, 2, 3-triol
(v) 2 – Methylphenol
(vi) 4 – Methylphenol
(vii) 2, 5 – Dimethylphenol
(viii) 2, 6 – Dimethyiphenol
(ix) 1 -Methoxy – 2 – methy lpropane
(x) Ethoxybenzene
(xi) 1 – Phenoxyheptane
(xii) 2 – Ethoxybutane.
Question 2. Write the structure of the compounds, whose IUPAC names are as follows
(i) 2-Methylbutan – 2 – ol
(ii) I-Phenylpropan – 2 – ol
(iii) 3-Phenylhexane – l, 3, 5 – triol
(iv) 2, 3 – Diethylphenol
(v) 1 – Ethoxypropane
(vi) 2- Ethoxy – 3- methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpentan – 3 – ol
(ix) Cyclopent – 3 – en – – ol
(x) 3 – Chloromethvlpentan – l – ol
Answer: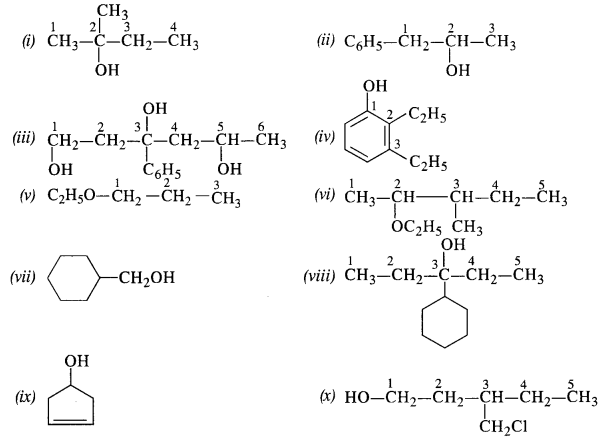
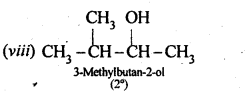
Question 3. (i) Draw the structures of all isomeric alcohols of molecular formula C5HI20 and give their IUPAC names.
(ii) Classify the isomers of alcohols in question 11.3 (i)as primary, secondary, and tertiary alcohols.
Answer: Eight isomers are possible. These are: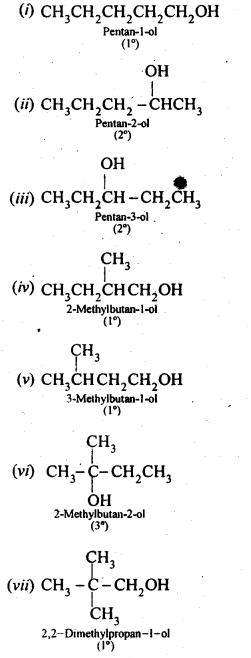
Question 4. Explain why is propanol higher boiling than butane?
Answer: Propanol (Propan-l-ol) and butane are of comparable molecular masses 60m and 58u respectively but the boiling point of propanol is higher (391 K) because of the presence of intermolecular hydrogen bonding in the molecules. However, it is not present in butane due to the absence of polar OH group. The only attractive forces are weak van der Waals forces. Therefore, the boiling point of propanol is more than that of butane (309 K).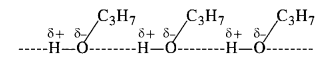
Question 5: Explain why are alcohols more soluble in water than the corresponding hydrocarbons?
Answer: The solubility of alcohols in water may be attributed to two factors :
(i) Both of them are of polar nature.
(ii) Molecules of both of them are involved in the intermolecular hydrogen bonding.
However, hydrocarbons are non-polar and are also not involved in any hydrogen bonding with alcohols. Alcohols readily dissolve in a water while the hydrocarbons are almost insoluble.
Question 6. What is meant by hydroboration oxidation reaction ? Illustrate with an example.
Answer: By hydroboration- oxidation of alkenes. Indirect hydration of alkenes can also bedone by hvdroboration-oxidation which is completed in two steps. In the first step. alkene reacts ith diborane (B’1{6) as boron hydride (BH3) dissolved in tetrahydrofuran (THF) to form an alkyl horane. In fact. the boron atom along i th the hydrogen gets attached to the double
bonded carbon atom with more number of hydrogen atoms less sterically hindered side). One hydrogen is then transferred to the other carbon atom. In this manner, all the three hydrogen atoms of boron are transferred to alkene molecule to form
trialkyl borane as the product. In the next step. the alkyl borane is oxidised by alkaline 11202 to form an alcohol. The indirect hydration proceeds according to Antimarkownikov s rule. For example.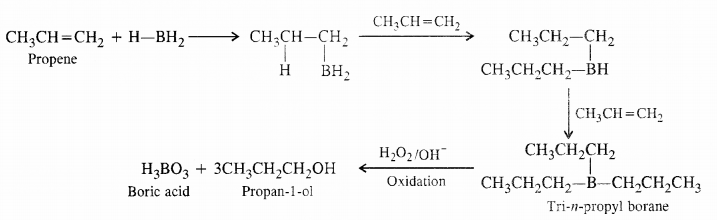
Question 7. Give the structures and IUPAC names of the phenols of molecular formula C7H8O.
Answer: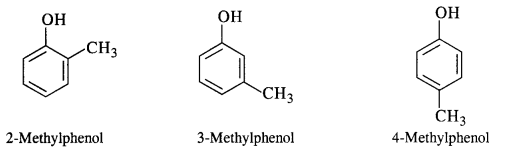
Question 8. In separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which is steam volatile? Give reason.
Answer: o-nitrophenol is steam volatile while p-nitrophenol is not. This is on account of intramolecular hydrogen bonding in the molecules of o-nitrophenol. As a result, its boiling point is less than that of p-nitrophenol in which the molecules are linked by intermolecular hydrogen bonding.
It is interesting to note that in the substituted phenols, the nature and position of the substituent influences the boiling point of phenol.
For example .o-nitrophenol is steam volatile while p-nitrophenol is not. This is supported by the fact that the boiling point temperature of o-nitrophenol (100°C) is less than that of p-nitrophenol, (279°C). In o-nitrophenol, there is intramolecular hydrogen bonding in OH and NO2 groups placed in a adjacent positions. However, these are linked by intermolecular hydrogen bonding in the p-isomers. It is quite obvious that extra energy is needed to cleave the hydrogen bonds in the p-isomer. Consequently, its boiling point is more.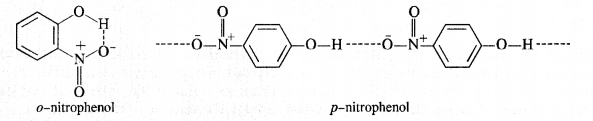
o-nitrophenol with lower boiling point is steam volatile while p-nitrophenol is not. This helps in the separation of the two isomers present in the liquid mixture. On passing steam, o-nitrophenol volatilises, and its vapours rise alongwith steam and after condensation, collect in the receiver p-nitrophenol is left behind in the distillation flask. e-nkrophenol p-nnrophenol.
Question 9. Give the equations of reactions for the preparation of phenol from cumene.
Answer: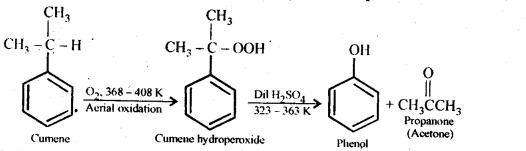
Question 10. Write chemical equations involved in the preparation of phenol from chlorobenzene.
Answer: From chlorobenzene, phenol is prepared by Dow’s process.In this method, chlorobenzene is heated with 6 to 8% solution of NaOH to about 623 K under a pressure of 300 atmospheres to form sodium phenate which upon acidification with dilute HCI gives phenol as follows: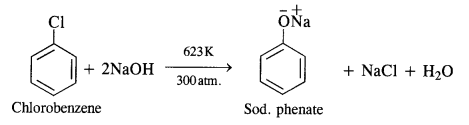
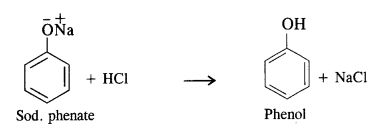
Question 11. Write the mechanism of hydration of ethene to yield ethanol.
Answer: Ethene does not react with water as such. Water being little polar, is not in a position to provide H+ ion for initial electrophilic attack on ethene. The reaction is carried in the presence of H2S04. The acid provides proton (H+) for the initial electrophilic attack.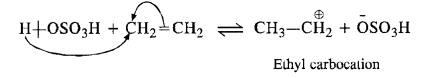
In the second step, H20 attacks the carbocation in preference to HS04 ion as a nucleophile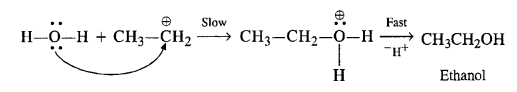
Question 12. You are given benzene, cone. H2S04 and NaOH. Write equations for the preparation of phenol using these reagents.
Answer: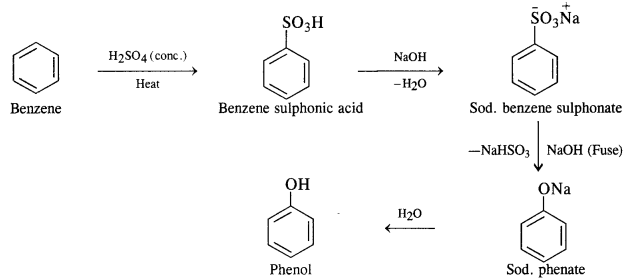
Question 13. Show how well you synthesise:
(i) 1-phenyl ethanol from a suitable alkene
(ii) Cyclohexylmethanol using an alkyl halide by SN² reaction
(iii) Pentan-1-ol using a suitable alkyl halide.
Answer: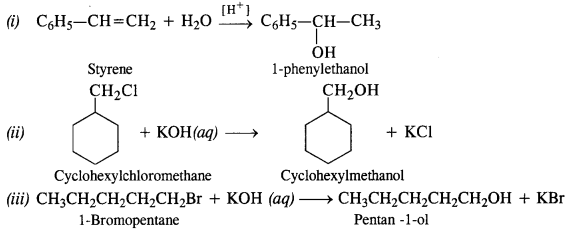
Question 14. Give two reactions to show the acidic nature of phenol. Compare the acidity of phenol with that of ethanol.
Answer: Acidic nature of phenols. Phenols are weakly acidic in nature. The liquid form of phenol containing about 5 percent water is known as carbolic acid. The dissociation constant (Ka) for phenol is 10-10 at 298 K (room temperature). The corresponding pKa* value is 10.0. The acidic character is exhibited by the following properties:
(i) Reaction with active metals. Phenols evolve hydrogen with active metals such as sodium and potassium.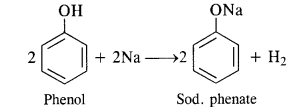
(ii) Reaction with alkalies. Phenols neutralise caustic alkalies such as NaOH or KOH to form salt and water.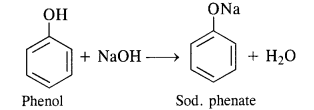
In addition to these, phenols turn blue litmus red which is the characteristic property or acids. However, phenols do not
react with either alkali metal carbonates or bicarbonates since these are quite weak acids.
Question 15. Explain why is ortho-nitrophenol more acidic than ortho-methoxy phenol?
Answer: Due to strong -R and – I-effect of the -NO2 group, electron density of the O – H bond decreases and hence the loss of a proton becomes easy.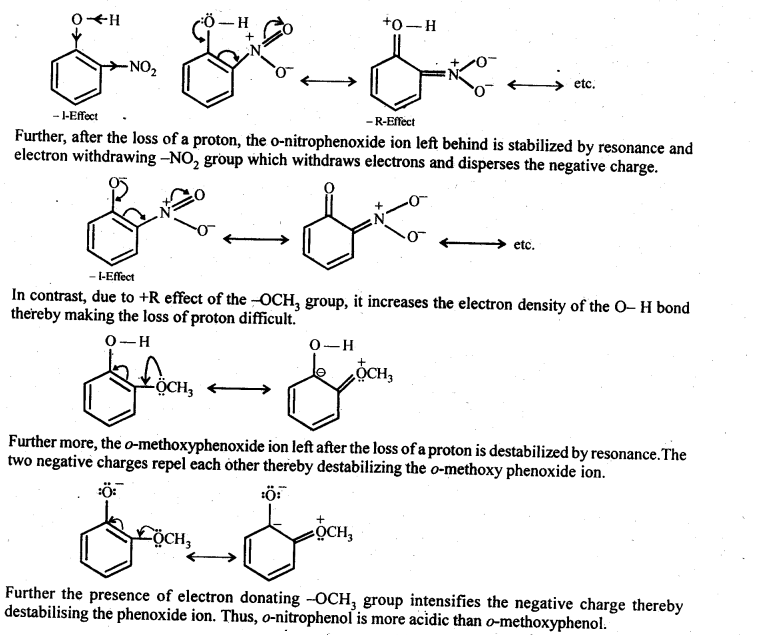
Question 16. Explain how does -OH group attached to a carbon atom of benzene ring activates it towards electrophilic substitution ?
Answer: The OH group exerts a +M (or + R) effect on the ring under the influence of attacking electrophile.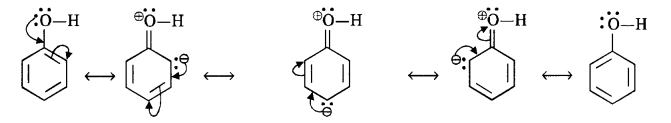
As a result, there is an increase in the electron density in the ring particularly at the ortho and para positions. The electrophilic substitution readily takes place at these positions when electrophile attacks.
Question 17. Give equations for the following chemical reactions :
(i) Oxidation of propan-1-ol with alkaline KMnO4
(ii) Reaction of bromine in CS2 with phenol
(iii) Action of dilute HNO3 on phenol
(iv) Treating phenol with chloroform in the presence of aqueous NaOH at 343 K.
Answer: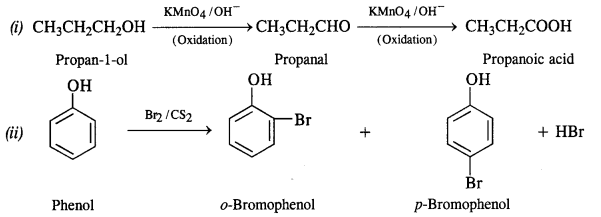
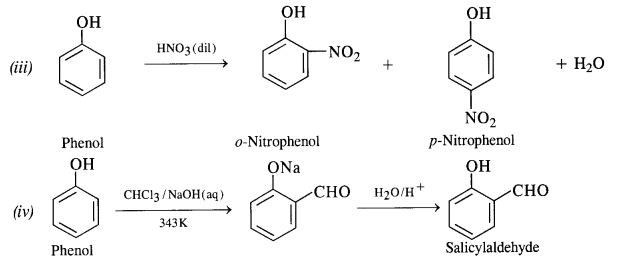
Question 18. Write short notes on (i) Kolbe reaction (ii) Reimer-Tiemann reaction.
Answer:
(i) In this reaction phenol is heated with chloroform alongwith aqueous NaOH (or KOH) to about 340 K. This is followed by acidification with dilute HCl when 2 – hydroxyhenzaldehyde (salicylaldehyde) is formed as the major product.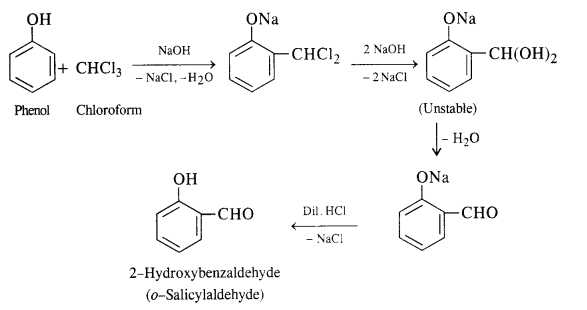
A small amount of para isomer is also formed in the reaction. In case, chLoroform is replaced by carbon tetrachloride, then 2—hydroxybenzoic acid (salicylic acid) is formed as the main product.
(ii) In this reaction, CO2 gas is passed through sodium phenate at 400 K under a pressure of 4 to 7 atmospheres. This is followed by acidification with dilute HCl when salicylic acid is formed. This method is commonly used for the commercial preparation of salicylic acid.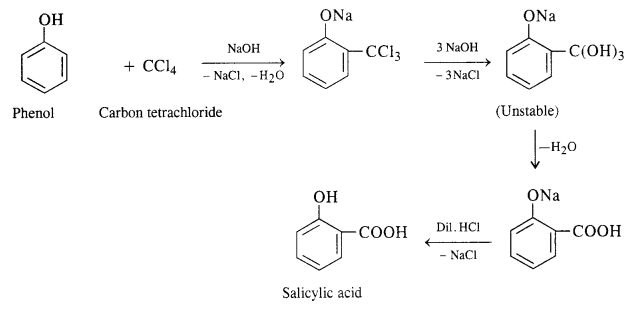
Question 19. Write a mechanism for the acid dehydration of ethanol to ethene.
Answer: Mechanism of dehydration. The mechanism is illustrated with ethanol which is a primary alcohol.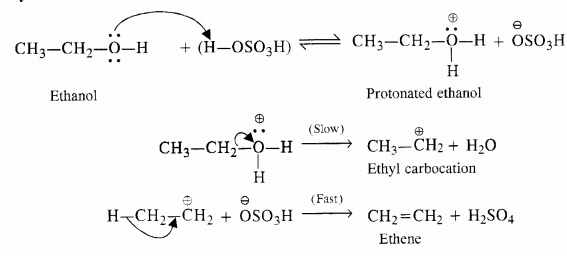
The relative ease of dehydration of different alcohols i.e. primary, secondary and tertiary can be further justified on the basis of the relative stabilities of the carbocations formed in the slow step. Since tertiary carbocation is maximum stable while primary is the least, the tertiary alcohols are maximum reactive while the primary are the least reactive in nature.
In other words, greater the stability of carbocation formed, more is the reactivity of the alcohol.
Question 20. How are the following conversions carried out?
(i) Propene → Propan -2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl magnesium chloride → Propan-1-ol
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol
Answer: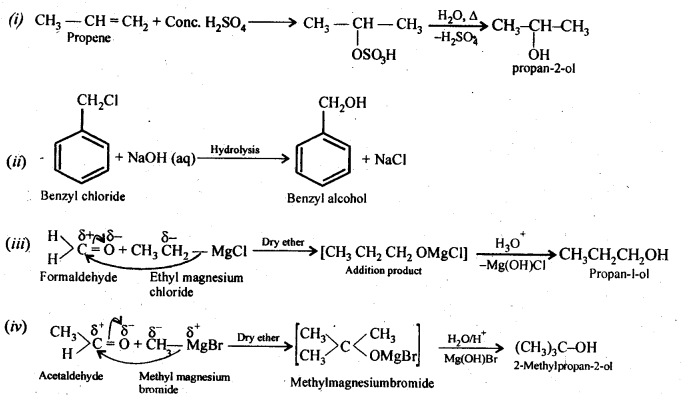
Question 21. Name the reagents used in the following reactions :
(i) Oxidation of primary alcohol to carboxylic acid
(ii) Oxidation of primary alcohol to an aldehyde
(iii) Bromination of phenol to 2, 4, 6-tribromophenol
(iv) Benzyl alcohol to benzoic acid
(v) Dehydration of propan-2-ol to propene
(vi) Butan-2-one to butan-2-ol.
Answer:
(i) Acidified K2Cr2O7
(ii) Pyridine chlorochromate (PCC)
(iii) Bromine water (Br2/H2O)
(iv) Alkaline KMnO4
(v) 60% H2S04 at 373 K
(vi) LiAlH4 or NaBH4.
Question 22. Give a reason for the higher boiling point of ethanol in comparison to methoxymethane.
Answer: Ethers are isomeric with alcohols but their boiling points are comparatively low due to the lack of hydrogen bonding. For example, boiling points of isomeric n – butyl alcohol (nC4H9OH) and diethyl ether (C2H5 – O – C2H5) are, 390 K and 308 K respectively.
Question 23: Give the IUPAC names of the following ethers :
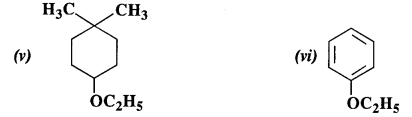
Answer:
(i) 1-Methoxy-2-methylpropane
(ii) 1-Chloro-2-methoxy ethane
(iii) 4-Nitroanisole
(iv) 1-Methoxypropane
(v) 4-Ethoxy-1, 1-dimethyl cyclohexane
(vi) Ethoxybenzene
Question 24. Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
(i) 1-Propoxypropane
(ii) Ethoxybenzene
(iii) 2-Methoxy-2-methylpropane
(iv) 1-Methoxyethane
Answer: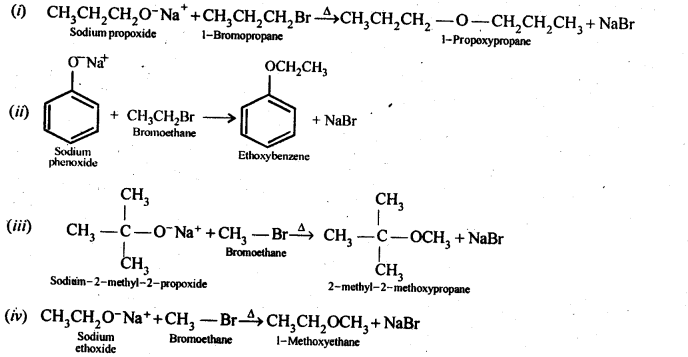
Question 25. Illustrate with examples the limitations of Williamson’s synthesis for the preparation of certain types of ethers.
Answer: Preparation from Alkyl Halides
From alkyl halides, ethers can be prepared by the following methods
By Williamson’s synthesis. It is the best method for the laboratory preparation of both simple and mixed ethers and involves the action of sodium alkoxide (formed by reaction between alcohol and sodium metal) on a suitable alkyl halide.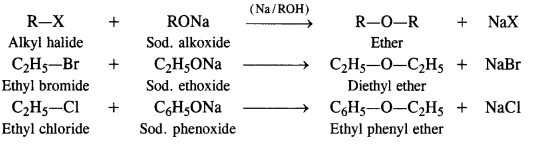
Limitations of the reaction. In the preparation of unsymmetrical ethers, a proper choice of the reactants is necessary.
Elimination leading to alkene can take place since alkoxide ion can also abstract one of the 3—hydrogen atom alongwith acting as a nucleophile. Thus, in order ro prepare ethyl tertiary butyl ether, we must use ethyl halide (primary) and sodium tertiary
butoxide.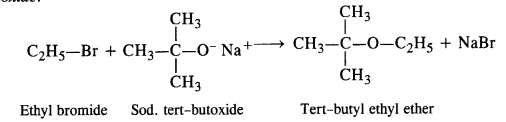
In case, the alkyl halide is tertiary and sodium ethoxide is employed, then C2H5O ion will cause the elimination of alkyl halide to form an alkene as the main product.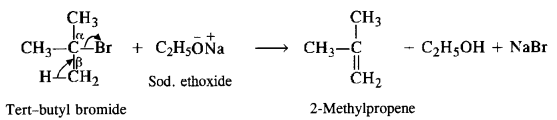
Since secondary and tertiary alkyl halides prefer to undergo elimination rather substitution, symmetrical ethers containing secondary and tertiary alkyl groups are obtained only in poor yields by Willamson’s synthesis. For example,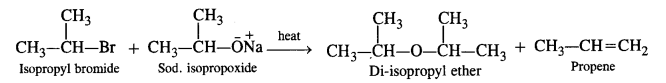
This method is also not successful for preparing aryl alkyl ethers by reacting sod. alkoxide with aryl halides because the cleavage of C – X bond is not so easy due to partial double bond character. In such cases, we must react sodium phenoxide with
alkyl halide as follows: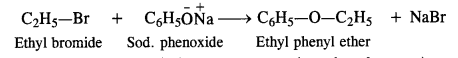
However, diaryl ethers (both the groups are aryl or phenyl groups) cannot be prepared since aryl halides fail to participate in the nucleophilic substitution reactions.
Question 26. How is 1-propoxypropane synthesised from propan-1-ol? Write the mechanism of this reaction.
Answer:
(a) Williamson’s synthesis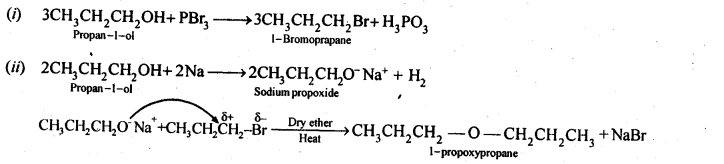
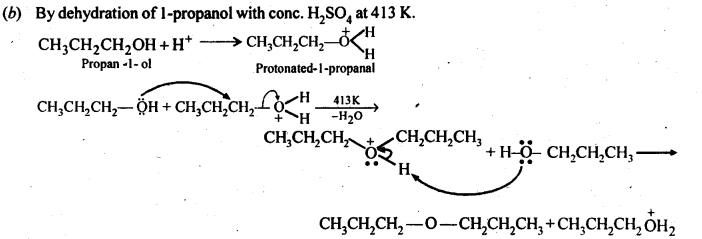
Question 27. Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Answer: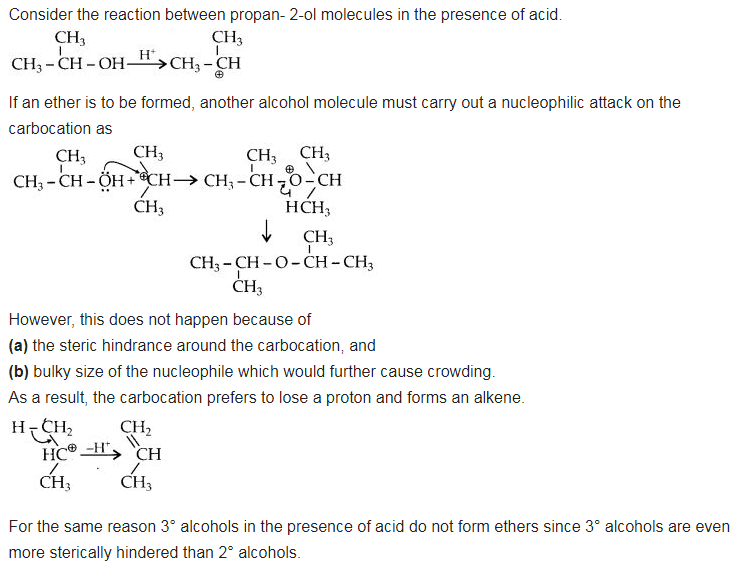
Question 28. Write the equation for the reaction of HI with :
(i) 1-Propoxypropane
(ii) Methoxybenzene
(iii) Benzyl ethyl ether.
Answer: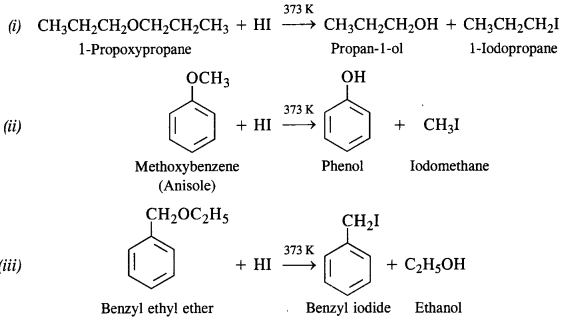
Question 29. Explain the fact that in aryl alkyl ethers (i) the alkoxy group activates the benzene ring towards electrophilic substitution and (ii) it directs the incoming substituents to ortho and para positions in benzene ring.
Answer: In aryl alkyl ethers, the +R-effect of the alkoxy (OR) group increases the electron density in the benzene ring, thereby activating the benzene ring towards electrophilic substitution reaction.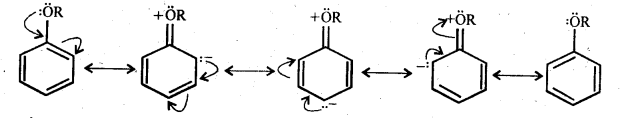
Since the electron density increases more at the two ortho and one para position as compared to meta position therefore, electrophilic substitution reactions mainly occur at o-and -positions.
Question 30. Write the mechanism of the reaction of Hl with methoxymethane.
Answer: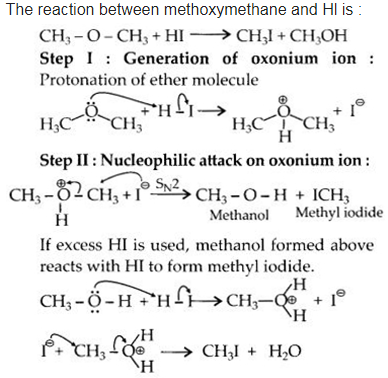
Question 31. Write equations for the following reactions :
(i) Friedel Crafts reaction-alkylation in anisole.
(ii) Nitration of anisole
(iii) Bromination of anisole in ethanolic medium
(iv) Friedel Crafts acetylation of anisole.
Answer:
(i) The halogenation of the benzene ring occurs at the ortho and para positions. However, the para isomer is
formed in excess. For example, the bromination of anisole in ethanoic acid gives nearly 90 percent p-bromoanisole.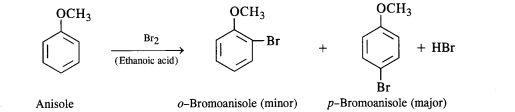
(ii) The nitration of anisole carried with a nitrating mixture of conc. UNO3 and conc. H2SO4 upon heating gives a
a mixture of ortho and para nitro derivatives.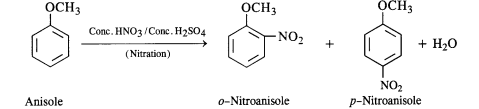
(iii) Sulphonation: Anisole upon sulphonation gives a mixture of isomeric sulphonic acid derivatives.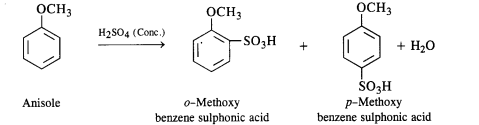
(iv) Friedel Craft’s reaction: Both alkylation and acylation are carried in the presence of anhydrous Aid3 catalyst which
behaves as a Lewis acid.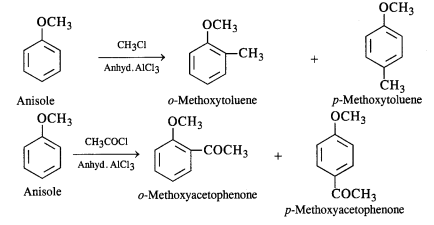
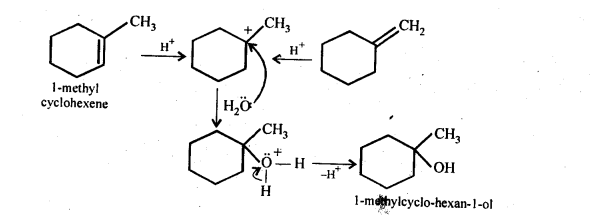
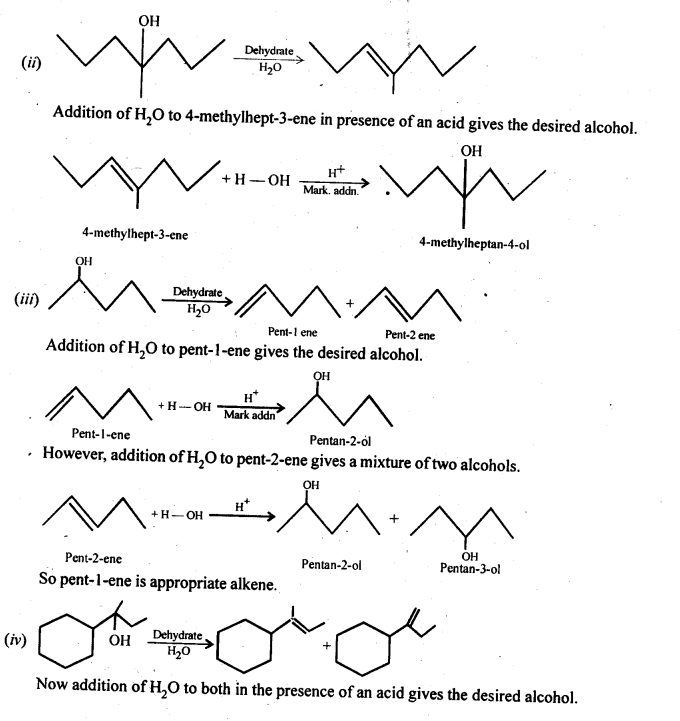
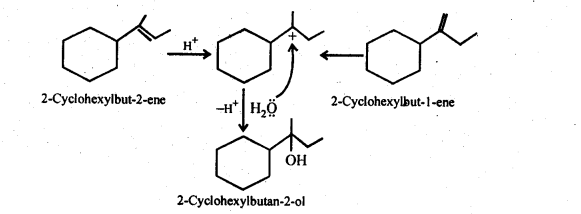
Question 32. Show how would you synthesise the following alcohols from appropriate alkanes?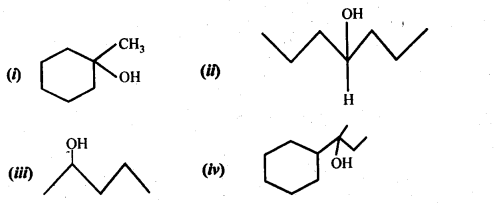
Answer: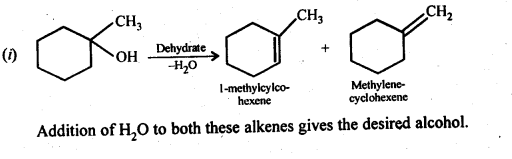
Question 33. When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place :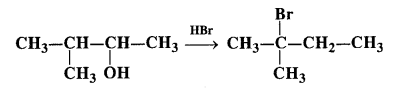
Give the mechanism of the reaction.
Answer: The mechanism is explained as follows :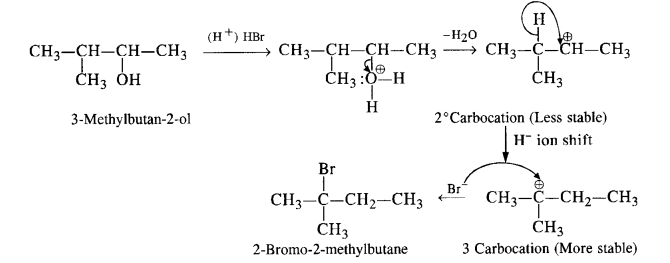

0 comment
Post a Comment