NCERT Solutions for Class-12 Chemistry Chapter-14 Biomolecules
NCERT INTEXT QUESTIONS
Question 1. Glucose and sucrose are soluble in water but cyclohexane and benzene (simple six-membered ring compounds) are insoluble in water. Explain.
Answer: Both glucose (C6H12O6) and sucrose (C12H22O11) are organic compounds and are expected to be insoluble in water. But quite surprisingly, they readily dissolve in water. This is due to the presence of a number of OH groups (five in the case of glucose and eight in sucrose) which are of polar nature. These are involved in the intermolecular hydrogen bonding with the molecules of H2O (water). As a result, both of them readily dissolve in water.
Benzene (C6H6) and cyclohexane (C6H12) are hydrocarbons which don’t have any polar group. They, therefore, don’t dissolve in water since there is hardly any scope of hydrogen bonding in their molecules with those of H2O (water).
Question 2. What are the expected products of hydrolysis of lactose?
Answer: The hydrolysis of lactose (disaccharide) can be done either with dilute HC1 or with enzyme emulsin. D-glucose and D-galactose are the products of hydrolysis. Both of them are monosaccharides with the molecular formula C6Hi206.
Question 3. How do you explain the absence of an aldehydic group in the pentaacetate of D-glucose?
Answer: Glucose, as we know is an aldohexose and it is expected to give the characteristic reactions of the aldehydic group e.g., action with NH2OH, HCN, Tollen’s reagent, Fehling reagent etc. However, the pentadactyl glucose formed by the acylation of glucose with acetic anhydride does not give these reactions.
This means that the aldehydic group is either absent or is not available in the penta acetyl glucose for chemical reactions. In fact, the aldehydic group is a part of the hemiacetal structure which the penta acetyl derivative has. It is, therefore, not free or available to take part in these reactions.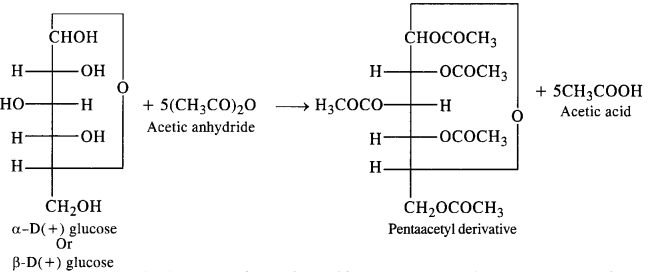
Question 4. The melting points and solubility of amino acids in water are generally higher than those of corresponding haloacids. Explain.
Answer: The amino acids exist as zwitter ions, H3N+ — CHR-COO-. Due to this dipolar salt like character, they have strong dipole-dipole attractions. Therefore, their melting points are higher than corresponding haloacids which do not have salt-like character.
Due to their salt-like character, amino acids interact strongly with water. As a result, their solubility in water is higher than corresponding haloacids which do not have a salt-like character.
Question 5. Where does the water in the egg go after boiling the egg?
Answer: Upon boiling the egg, denaturation of globular protein present in it occurs. Water present probably gets either absorbed or adsorbed during denaturation and disappears.
Question 6. Explain why vitamin C can not be stored in the body.
Answer: Vitamin C is mainly ascorbic acid which is water-soluble. It is readily excreted through urine and cannot be stored in the body.
Question 7. What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Answer: The products obtained are 2-deoxy-D-ribose, phosphoric acid, and thymine.
Answer: As we know, a molecule of DNA has a double-strand structure, and the four complementary bases pair each other. Cytosine (C) always pairs up with guanine (G) while thymine (T) is paired up with adenine (A). Because of the presence of the double-strand structure, when a molecule of DNA is hydrolysed, in each pair the molar ratio of the bases remains the same. However, this is not seen when RNA is subjected to hydrolysis. This suggests that RNA has not a double-strand structure like DNA. It exists as a single strand.
NCERT EXERCISE
Question 1. What are monosaccharides?
Answer: Monosaccharides are carbohydrates which cannot be hydrolysed to smaller molecules. Their general formula is (CH2O)n where n = 3 → 7. These are of two types. Those which contain an aldehyde (-CHO) group are called aldose and those which contain a keto (C = 0) group are called ketose. They are further classified as triose, tetrose, pentose etc. according to the no. of carbon atoms present (3, 4, 5 respectively).
Question 2. What are reducing sugars?
Answer: Carbohydrates which reduce Fehling’s solution to red precipitate of Cu20 or Tollen’s reagent to metallic Ag are called reducing sugars. All monosaccharides (both aldoses and ketoses) and disaccharides except sucrose are reducing sugars. Thus, D – (+) – glucose, D-(-)-fructose, D – (+) – maltose and D – (+) – lactose are reducing sugars.
Answer:
1. Structural material for plant cell walls: The polysaccharides cellulose acts as the chief structural material of the plant’s cell walls.
2. Reserve food material: The polysaccharide starch is the major reserve food material in the plants. It is stored in seeds and acts as the reserve food material for the tiny plant till its capable of making food on its own by photosynthesis.
Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Answer:
Monosaccharides: Ribose, 2-deoxyribose, galactose, fructose.
Disaccharides: Maltose, lactose.
Question 5. What do you understand by the term glycosidic linkage?
Answer: Glycosidic linkage is used to link different monosaccharides in disaccharides and polysaccharides through an oxygen atoms. For example, the glycosidic linkage is present in sucrose, lactose, maltose, etc. These are all disaccharides.
Answer:
1. The carbohydrates are stored in the animal body as glycogen. It is present in the liver, muscles, and brain. Enzymes break the glycogen down to glucose when the body needs glucose.
2. Glycogen is more highly branched than amylopectin (starch) glycogen chain consists of 10-14 glucose units, whereas amylopectin (starch) glycogen chain consists of 20-25 glucose units.
Question 7. What are the hydrolysis products of starch and lactose?
Answer: Starch upon hydrolysis gives α – D(+) glucose which is the constituent of both amylose and amylopectin. Lactose upon hydrolysis gives galactose and glucose.
Upon hydrolysis, cellulose gives only D(+) glucose. This means that only D(+) glucose units are present in cellulose but unlike starch these are -D(+) glucose molecules and not a – D(+) glucose molecules. The X – ray analysis has shown that there are large linear chains of 3 – D( +) glucose molecules lying side by side in the form of bundles held together by hydrogen bonding in the neighbouring hydroxyl groups.
Question 8. What is the basic structural difference between starch and cellulose?
Answer: Starch is not a single component. It consists of amylose and amylopectin. In contrast, cellulose is a single compound. Amylose is a linear polymer of α – D glucose while cellulose is a linear polymer of β -D glucose. In amylose, C1 – C4 α- glycosidic linkage is present, whereas in cellulose C1 – C4 β- glycosidic linkage is present. Amylopectin has a highly branched structure.
Question 9. What happens when D-glucose is treated with
(i) HI
(ii) Bromine water
(iii) HNO3?
Answer:
(i) Reaction with HI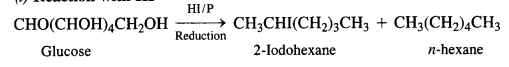
(ii) Reaction with bromine water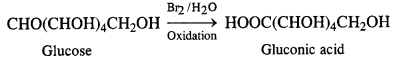
(iii) Reaction with HNO3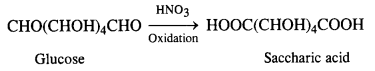
Answer: The following reactions of D- glucose cannot be explained by its open-chain structure.
1. D – the glucose does not undergo certain characteristic reactions of aldehydes. For example, glucose does not form NaHSO3 addition product, aldehyde-ammonia adduct, 2, 4, DNP derivative and does not respond to Schiff’s reagent test.
2. Glucose reacts with NH2OH to form an oxime but glucose pentaacetate does not. This implies that the aldehyde group is absent in glucose pentaacetate.
3. D (+) – Glucose exists in two stereoisomeric forms ie. α – glucose and β- glucose, α – D (+) – glucose is obtained when a concentrated aqueous or alcoholic solution is crystallised at 303K. It has a melting point of 419K and has a specific rotation of +111° in a freshly prepared aqueous solution. However when glucose is crystallised from the water above 371 K β – D (+) glucose is obtained.
4. Both α – D glucose and β – D glucose undergoes mutarotation in an aqueous solution.
Question 11. What are essential and non-essential amino acids?
Answer: α – Amino acids which are needed for the health and growth of human beings but are not synthesised by the human body are called essential amino acids. For example, valine, leucine phenylalanine etc. On the other hand α – amino acids which are needed for the health and growth of human beings and are synthesised by the human body are called non-essential amino acids. For example glycine, alanine, aspartic acid etc.
Question 12. Define the following as related to proteins
(i) Peptide linkage
(ii) Primary structure
(iii) Denaturation.
Answer:
(i) Peptide Linkage: Proteins are the polymers of a-amino acids which are connected to each other by peptide bond or peptide linkage. Chemically, peptide linkage is an amide formed between -COOH group and -NH2 group. The reaction between two molecules of similar or different amino acids, proceeds through the combination of the amino group of one molecule with the carboxyl group of the other This results in the elimination of a water molecule and formation of a peptide bond -CO-NH-. The product of the reaction is called a dipeptide because it is made up of two amino acids. For example, when carboxyl group of glycine combines with the amino group of alanine we get a dipeptide, glycylalanine.
(ii) Primary Structure: Proteins may have one or more polypeptide chains. Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is said to be the primary structure of that protein. Any change in this primary structure i.e.; the sequence of amine acids creates a different protein.
(iii) Denaturation: Protein found in a biological system with a unique three-dimensional structure and biological activity is called a native protein. When a protein in its native form, is subjected to physical change like change in temperature or chemical change in pH, the hydrogen bonds are disturbed. Due to this; globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein. During denaturation 2° and 3° structures are destroyed but 1° structure remains intact. The coagulation of egg white on boiling is a common example of denaturation. Another example is the curdling of milk which is caused due to the formation of lactic acid by the bacteria present in milk.
Answer: Secondary structure of a protein refers to the shape in which a long polypeptide chain can exist. These are found to exist in two types :
1. α-helix structure
2. β-pleated sheet structure.
Secondary Structure of Proteins:
The long, flexible peptide chains of proteins are folded into the relatively rigid regular conformations called the
secondary structure. It refers to the conformation which the polypeptide chains assume as a result of hydrogen bonding
between the > C= O and > N-H groups of different peptide bonds.
The type of secondary structure a protein will acquire, in general, depends upon the size of the R-group. If the size of the
R-groups are quite large, the protein will acquire a ct-helix structure. If on the other hand, the size of the R-groups are relatively
smaller, the protein will acquire a β – flat sheet structure.
(a) α-Helix structure: If the size of the R-groups is quite large, the hydrogen bonding occurs between > C = O group
of one amino acid unit and the > N-H group of the fourth amino acid unit within the same chain. As such the polypeptide
chain coils up into a spiral structure called right-handed ct- helix structure. This type of structure is adopted by most of the
fibrous structural proteins such as those present in wool, hair, and muscles. These proteins are elastic i.e., they can be
stretched. During this process, the weak hydrogen bonds causing the α- helix are broken. This tends to increase the length of
the helix like a spring. On releasing the tension, the hydrogen bonds are reformed, giving back the original helical shape.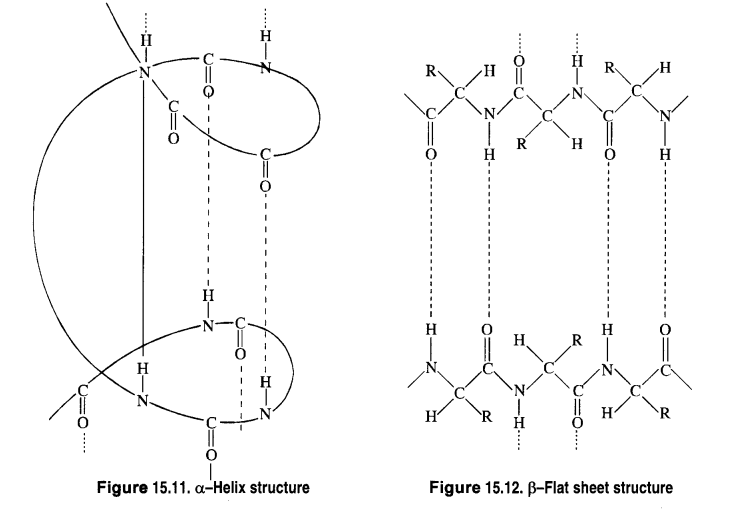
(b) β—Flat sheet or β—Pleated sheet structure: If R-groups are relatively small, the peptide chains lie side by side in a zig
zag manner with alternate R-groups on the same side situated at fixed distances apart. The two such neighbouring chains are held together by intermolecular hydrogen bonds. A number of such chains can be inter-bonded and this results in the formation of a flat sheet structure These chains may contract or bend a little in order to accommodate moderate-sized R-groups. As a result, the sheet bends into parallel folds to form a pleated sheet structure known as β – pleated sheet structure. These sheets are then stacked one above the other like the pages of a book to form a three-dimensional structure. The protein fibrion in silk fibre has a β – pleated sheet structure. The characteristic mechanical properties of silk can easily be explained on the basis of its β – sheet structure. For example, silk is non-elastic since stretching leads to pulling the peptide covalent bonds. On the other hand, it can be bent easily like a stack of pages because, during this process, the sheets slide over each other.
Question 14. What type of bonding helps in stabilising the α-helix structure of proteins?
Answer: The α-helix structure of proteins is stabilized by intramolecular H-bonding between C = O of one amino acid residue and the N – H of the fourth amino acid residue in the chain. This causes the polypeptide chain to coil up into a spiral structure called the right-handed α- helix structure.
Question 15. Differentiate between globular proteins and fibrous proteins. (Jharkhand Board 2014; C.B.S.E. Delhi 2015)
Answer:
| Globular proteins | Fibrous proteins |
| 1. Polypeptide chains are arranged as coils. | 1. Polypeptide chains run parallel to each other. |
| 2. They have a spherical shape. | 2. They have a thread-like structure. |
| 3. These are water-soluble. | 3. These are insoluble in water. |
| 4. These are sensitive to a small change in temperature and pH. | 4. These are not affected by a small change in temperature and pH. |
| 5. They possess biological activity. | 5. They don’t have any biological activity but serve as the chief structural material of animal tissues. |
Question 16. How do you explain the amphoteric behaviour of amino acids?
Answer: Amino acids have basic (NH2) and acidic (COOH) groups. These are, therefore, amphoteric in nature. However, they exhibit these characters in the amino acid molecule itself i.e., NH2 group (basic) accepts a proton from COOH group (acidic) in the same molecule. Therefore, a-amino acid exists as a dipolar ion.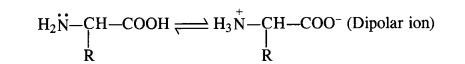
Question 17. What are enzymes?
Answer: We have learned in the study of carbohydrates that these are body fuels i.e., they provide the necessary energy to the body and
keeps it working. Actually, the human body is just like a furnace in which chemical reactions take place and are responsible for the digestion of food, absorption of appropriate molecules, and production of energy. The entire process involves a series of reactions that are catalyzed by biocatalysts known as enzymes. Thus, enzymes may be defined as:
Biological or Biocatalysts which catalyse the reactions in living beings.
All enzymes are basically globular proteins. Enzymes are very specific for a particular reaction as well as for a particular
substrate. These are generally named after the compounds or the class of substances with which they are linked or work.
Question 18. What is the effect of denaturation on the structure of proteins?
Answer: As a result of denaturation, globules get unfolded and helixes get uncoiled. Secondary and tertiary structures of the protein are destroyed, but the primary structures remain unaltered. It can be said that during denaturation, secondary and tertiary – structured proteins get converted into primary – structured proteins. Also, as the secondary and tertiary structures of a protein are destroyed, the enzyme loses its activity.
Question 19. How are vitamins classified? Name the vitamin responsible for the coagulation of blood? (C.B.S.E. Outside Delhi 2015)
Answer: On the basis of their solubility in water or fat, vitamins are classified into two groups:
1. Fat-soluble vitamins:
Vitamins that are soluble in fat and oils, but not in the water, belong to their group.
For example, Vitamins A, D, E, and K.
2. Water-soluble vitamins:
Vitamins that are soluble in water belong to their group.
For example, B group vitamins (B1, B2, B6, B12, etc) and vitamin C.
However, biotin or vitamin H is neither soluble in water nor in fat.
Vitamin K is responsible for the coagulation of blood.
Answer:
Vitamin A: Soluble in water but insoluble in oils and fas. Destroyed by cooking or prolonged exposure to air. it increases the resistance of the body towards diseases. Maintains healthy skin and helps in the healing of cuts and abrasions.
Vitamin C: Soluble in oils and fats but insoluble in water, stable to heat. Promotes growth and improves vision. ¡t also increases resistance to disease. It is available in Citrus fruits (oranges, lemon, grapefruit, lime, etc.), amia, cabbage. guava etc.
Answer: Nucleic acids are biologically important polymers which are present in all living cells.
1. Nucleic acids play a vital role in the transmission of heredity characteristics.
2. Nucleic acids help in the biosynthesis of proteins.
Two important biological functions of nucleic acids are (i) Replication and (ii) protein synthesis.
These are briefly discussed.
Replication: Replication may be defined as the process by which a single DNA molecule produces two identical copies of itself.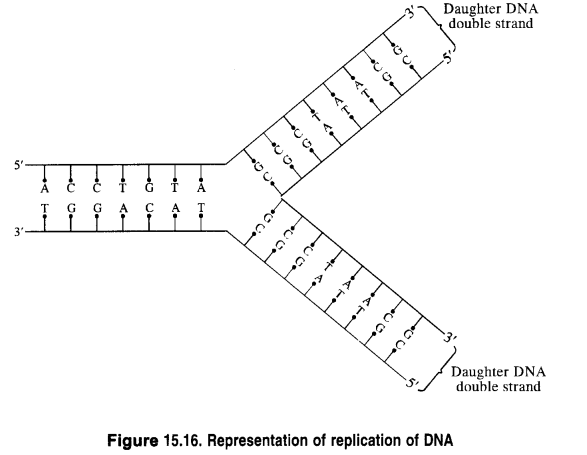
Replication is an enzyme-catalyzed process. The process of replication starts with the partial unwinding of the two strands of the DNA double helix through the breaking of the hydrogen bonds between pairs of bases. Each strand then acts as the template (or pattern) for the synthesis of two new strands of DNA in the celllix environment. The specificity of base-pairing ensures that each new strand is complementary to its old template strand. As a result, two identical copies of DNA from the original DNA are produced. Each of these two copies is then passed on to the two new cells resulting from cell division. In this way, hereditary characters are transmitted from one cell to another.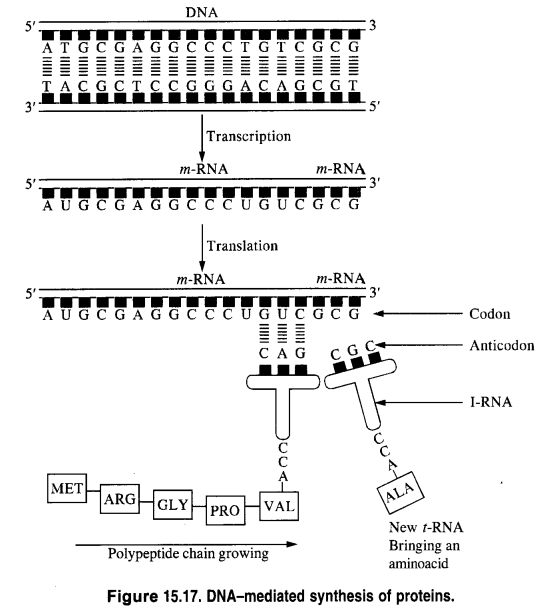
Question 22. What is the difference between a nucleoside and a nucleotide?
Answer: A nucleoside contains a pentose sugar and base (purine or pyrimidine) while in the nucleotide, a phosphoric acid component is also present.
Arrangement of constituents in Nucleic Acids. These are, intact, three building blocks in nucleic acid. A combination of base and sugar are known as a nucleoside. Similarly, base, sugar, and phosphates from nucleotides while nucleic acids are
polynucleotides which means that these are the polymers of nucleotides.
Question 23. The two strands in DNA are not identical but are complementary. Explain.
Answer: In the helical structure of DNA, the two strands are held together by hydrogen bonds between specific pairs of bases. Cytosine forms a hydrogen bond with guanine, while adenine forms a hydrogen bond with thymine. As a result, the two strands are complementary to each other.
Question 24. Write the important structural and functional differences between DNA and RNA.
Answer:
| Ribonucleic acid (RNA) | Deoxyribonucleic acid (DNA) |
| 1. The pentose sugar present in RNA is D-ribose | 1. The pentose sugar present in DNA is D-2-deoxyribose |
| 2. RNA contains cytosine and uracil as pyrimidine bases and guanine and adenine as purine bases. | 2. DNA contains cytosine and thymine as pyrimidine bases and guanine and adenine as purine bases. |
| 3. It is a single chain of polynucleotides. | 3. It is a double chain of polynucleotides. |
| 4. It is formed by DNA and cannot replicate itself. | 4. It can replicate itself. |
| 5. Its molecule is relatively short with low molecular mass. | 5. Its molecule is relatively long with a high molecular mass. |
| 6. It regulates protein synthesis. | 6. It controls structure, metabolism, differentiation and transfer the characters from one generation to the other. |
| 7. It is an essential genetic material of plant viruses. | 7. It is an essential genetic material of eukaryotic cells. |
Answer:
1. Messenger RNA (m – RNA)
2. Ribosomal RNA (r – RNA)
3. Transfer RNA (t – RNA)

0 comment
Post a Comment